VETAKET- ketamine hydrochloride injection, solution
VetaKet by
Drug Labeling and Warnings
VetaKet by is a Animal medication manufactured, distributed, or labeled by Akorn Animal Health, Inc., Akorn, Inc, Akorn, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- CAUTION:
-
DESCRIPTION:
Ketamine hydrochloride is a rapid-acting, nonnarcotic, nonbarbiturate agent for anesthetic use in cats and for restraint in subhuman primates. It is chemically designated dl 2-(o-chlorophenyl) - 2 - (methylamino) cyclohexanone hydrochloride and is supplied as a slightly acid (pH 3.5 to 5.5) solution for intramuscular injection in a concentration containing the equivalent of 100 mg ketamine base per milliliter and contains 0.1 mg/mL benzethonium chloride as a preservative.
-
ACTION:
Ketamine hydrochloride is a rapid-acting agent whose pharmacological action is characterized by profound analgesia, normal pharyngeal-laryngeal reflexes, mild cardiac stimulation and respiratory depression. Skeletal muscle tone is variable and may be normal, enhanced or diminished. The anesthetic state produced does not fit into the conventional classification of stages of anesthesia, but instead ketamine hydrochloride produces a state of unconsciousness which has been termed “dissociative” anesthesia in that it appears to selectively interrupt association pathways to the brain before producing somesthetic sensory blockade.
In contrast to other anesthetics, protective reflexes, such as coughing and swallowing, are maintained under ketamine hydrochloride anesthesia. The degree of muscle tone is dependent upon level of dose; therefore, variations in body temperature may occur. At low dosage levels there may be an increase in muscle tone and a concomitant slight increase in body temperature. However, at high dosage levels there is some diminution in muscle tone and a resultant decrease in body temperature, to the point where supplemental heat may be advisable.
In cats, there is usually some transient cardiovascular stimulation, increased cardiac output with slight increase in mean systolic pressure with little or no change in total peripheral resistance. At higher doses respiratory rate is usually decreased.
The assurance of a patent airway is greatly enhanced by virtue of maintained pharyngeal-laryngeal reflexes. Although some salivation is occasionally noted, the persistence of the swallowing reflex aids in minimizing the hazards associated with ptyalism. Salivation may be effectively controlled with atropine sulfate in dosages of 0.04 mg/kg (0.02 mg/lb) in cats and 0.01 to 0.05 mg/kg (0.005 to 0.025 mg/lb) in subhuman primates.
Other reflexes, e.g., corneal, pedal, etc., are maintained during ketamine hydrochloride anesthesia, and should not be used as criteria for judging depth of anesthesia. The eyes normally remain open with the pupils dilated. It is suggested that a bland ophthalmic ointment be applied to the cornea if anesthesia is to be prolonged.
Following administration of recommended doses, cats become ataxic in about 5 minutes with anesthesia usually lasting from 30 to 45 minutes at higher doses. At the lower doses, complete recovery usually occurs in 4 to 5 hours but with higher doses recovery time is more prolonged and may be as long as 24 hours.
In studies involving 14 species of subhuman primates represented by at least 10 anesthetic episodes for each species, the median time to restraint ranged from 1.5 [Aofus tnvirgatus (night monkey) and Cebus capucinus (white-throated capuchin)] to 5.3 minutes [Macaca nemestrina (pig-tailed macaque)]. The median duration of restraint ranged between 20 and 55 minutes in all but five of the species studied. Total time from injection to end of restraint ranged from 43 [Saimiri sciureus (squirrel monkey)] to 183 minutes [Macaca nemestrina (pig-tailed macaque)] after injection. Recovery is generally smooth and uneventful. The duration is dose related.
By single intramuscular injection, ketamine hydrochloride usually has a wide margin of safety in cats and subhuman primates. In cats, cases of prolonged recovery and death have been reported.
- INDICATIONS:
-
CONTRAINDICATIONS:
Ketamine hydrochloride is contraindicated in cats and subhuman primates suffering from renal or hepatic insufficiency.
Ketamine hydrochloride is detoxified by the liver and excreted by the kidneys; therefore, any preexistent hepatic or renal pathology or impairment of function can be expected to result in prolonged anesthesia; related fatalities have been reported.
-
PRECAUTIONS:
In cats, doses in excess of 50 mg/kg during any single procedure should not be used.
The maximum recommended dose in subhuman primates is 40 mg/kg.
To reduce the incidence of emergence reactions, animals should not be stimulated by sound or handling during the recovery period. However, this does not preclude the monitoring of vital signs.
Apnea, respiratory arrest, cardiac arrest, and death have occasionally been reported with ketamine used alone, and more frequently when used in conjunction with sedatives or other anesthetics. Close monitoring of patients is strongly advised during induction, maintenance, and recovery from anesthesia.
-
ADVERSE REACTIONS:
Respiratory depression may occur following administration of high doses of ketamine hydrochloride. If at any time respiration becomes excessively depressed and the animal becomes cyanotic, resuscitative measures should be instituted promptly. Adequate pulmonary ventilation using either oxygen or room air is recommended as a resuscitative measure.
Adverse reactions reported have included emesis, salivation, vocalization, erratic recovery and prolonged recovery, spastic jerking movements, convulsions, muscular tremors, hypertonicity, opisthotonos, dyspnea, and cardiac arrest. In the cat, myoclonic jerking and/or mild tonic convulsions can be controlled by ultrashort-acting barbiturates which should be given to effect. The barbiturates should be administered intravenously at a dose level of one-sixth to one-fourth the usual dose for the product being used. Acepromazine may also be used. However, recent information indicates that some phenothiazine derivatives may potentiate the toxic effects of organic phosphate compounds such as found in flea collars and certain anthelmintics. A study has indicated that ketamine hydrochloride alone does not potentiate the toxic effects of organic phosphate compounds.
-
DOSAGE AND ADMINISTRATION:
Ketamine hydrochloride is well tolerated by cats and subhuman primates when administered by intramuscular injection.
Fasting prior to induction of anesthesia or restraint with ketamine hydrochloride is not essential; however, when preparing for elective surgery, it is advisable to withhold food for at least six hours prior to administration of ketamine hydrochloride.
Anesthesia may be of shorter duration in immature cats. Restraint in subhuman primate neonates (less than 24 hours of age) is difficult to achieve.
As with other anesthetic agents, the individual response to ketamine hydrochloride is somewhat varied depending upon the dose, general condition, and age of the subject so that dosage recommendations cannot be absolutely fixed.
-
DOSAGE:
Cats: A dose of 11 mg/kg (5 mg/lb) is recommended to produce restraint. Dosages from 22 to 33 mg/kg (10 to 15 mg/lb) produce anesthesia that is suitable for diagnostic or minor surgical procedures that do not require skeletal muscle relaxation.
Subhuman primates: The recommended restraint dosages of ketamine hydrochloride for the following species are: Cercocebus torquatus (white-collared mangabey), Papio cynocephalus (yellow baboon), Pan troglodytes verus (chimpanzee), Papio anubis (olive baboon), Pongo pygmaeus (orangutan), Macaca nemestrina (pig-tailed macaque) 5 to 7.5 mg/kg; Presbytis entellus (entellus langur) 3 to 5 mg/kg; Gorilla gorilla gorilla (gorilla) 7 to 10 mg/kg; Aotus trivirgatus (night monkey) 10 to 12 mg/kg; Macaca mulatta (rhesus monkey) 5 to 10 mg/kg; Cebus capucinus (white-throated capuchin) 13 to 15 mg/kg; and Macaca fascicularis (crab eating macaque), Macaca radiata (bonnet macaque), and Saimiri sciureus (squirrel monkey) 12 to 15 mg/kg.
A single intramuscular injection produces restraint suitable for TB testing, radiography, physical examination, or blood collection.
-
HOW SUPPLIED:
VetaKet (ketamine hydrochloride injection, USP) is supplied as the hydrochloride in concentrations equivalent to ketamine base.
Each 10 mL vial contains 100 mg/mL.
NDC: 59399-114-10 10 mL vial in packages of 25
NDC: 59399-114-12 10 mL vial in packages of 12
-
CLINICAL STUDIES:
Ketamine hydrochloride has been clinically studied in subhuman primates in addition to those species listed under Dosage and Administration. Dosages for restraint in these additional species, based on limited clinical data, are: Cercopithecus aethiops (grivet), Papio papio (guinea baboon) 10 to 12 mg/kg; Erythrocebus patas patas (patas monkey) 3 to 5 mg/kg; Hylobates lar (white-handed gibbon) 5 to 10 mg/kg; Lemur catta (ringtailed lemur) 7.5 to 10 mg/kg; Macaca fuscata (Japanese macaque) 5 mg/kg; Macaca speciosa (stumptailed macaque) and Miopithecus talapoin (mangrove monkey) 5 to 7.5 mg/kg; and Symphalangus syndactylus (siamangs) 5 to 7 mg/kg.
Taxonomy from “A Handbook of Living Primates” by Napier and Napier, Academic Press, New York, NY.
AKORN
Animal Health
Manufactured by:
Akorn, Inc.
Lake Forest, IL 60045VE00N Rev. 09/15
-
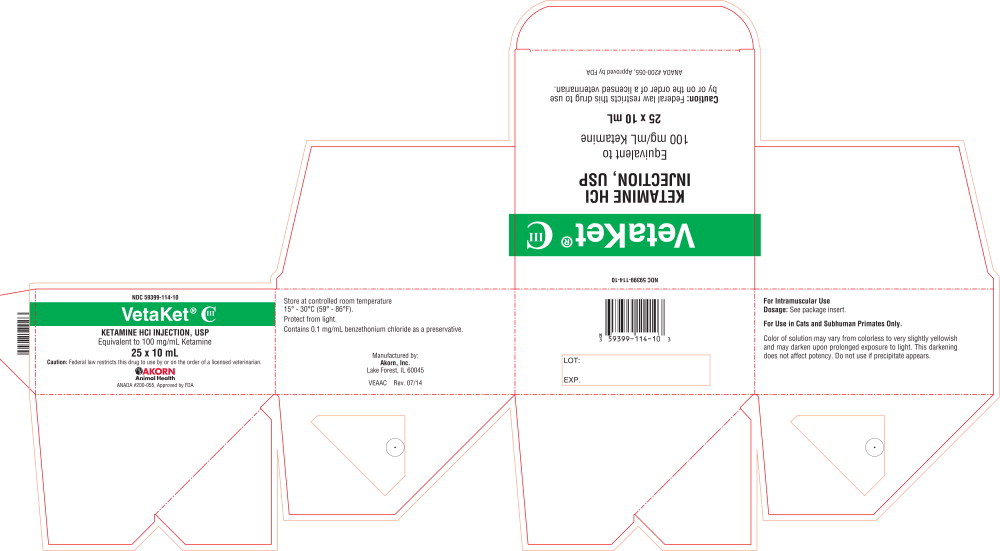
PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Container Label:
NDC: 59399-114-10
VetaKet® CIII
KETAMINE HCl
INJECTION, USP
Equivalent to
100 mg per mL Ketamine
10 mL
CAUTION: Federal law restricts this drug to use
by or on the order of a licensed veterinarian.
ANADA 200-055, Approved by FDA
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Carton Label:
NDC: 59399-114-10
VetaKet® CIII
KETAMINE HCl INJECTION, USP
Equivalent to 100 mg/mL Ketamine
25 x 10 mL
CAUTION: Federal law restricts this drug to use by or on the order of a licensed Veterinarian.
Akorn Animal Health Logo
ANADA 200-055, Approved by FDA
-
INGREDIENTS AND APPEARANCE
VETAKET
ketamine hydrochloride injection, solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 59399-114 Route of Administration INTRAMUSCULAR DEA Schedule CIII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Ketamine Hydrochloride (UNII: O18YUO0I83) (Ketamine - UNII:690G0D6V8H) Ketamine 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength Benzethonium Chloride (UNII: PH41D05744) 0.1 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59399-114-10 25 in 1 CARTON 1 10 mL in 1 VIAL, MULTI-DOSE 2 NDC: 59399-114-12 12 in 1 CARTON 2 10 mL in 1 VIAL, MULTI-DOSE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200055 03/09/2015 Labeler - Akorn Animal Health, Inc. (078876357) Establishment Name Address ID/FEI Business Operations Akorn, Inc 063434679 PACK, LABEL Establishment Name Address ID/FEI Business Operations Akorn, Inc. 155135783 MANUFACTURE, ANALYSIS, STERILIZE
Trademark Results [VetaKet]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 VETAKET 74266573 1803032 Live/Registered |
LLOYD, INC. OF IOWA 1992-04-17 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.