Alcohol Gel Hand Sanitizer by Kay Chemical Company Drug Facts
Alcohol Gel Hand Sanitizer by
Drug Labeling and Warnings
Alcohol Gel Hand Sanitizer by is a Otc medication manufactured, distributed, or labeled by Kay Chemical Company. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
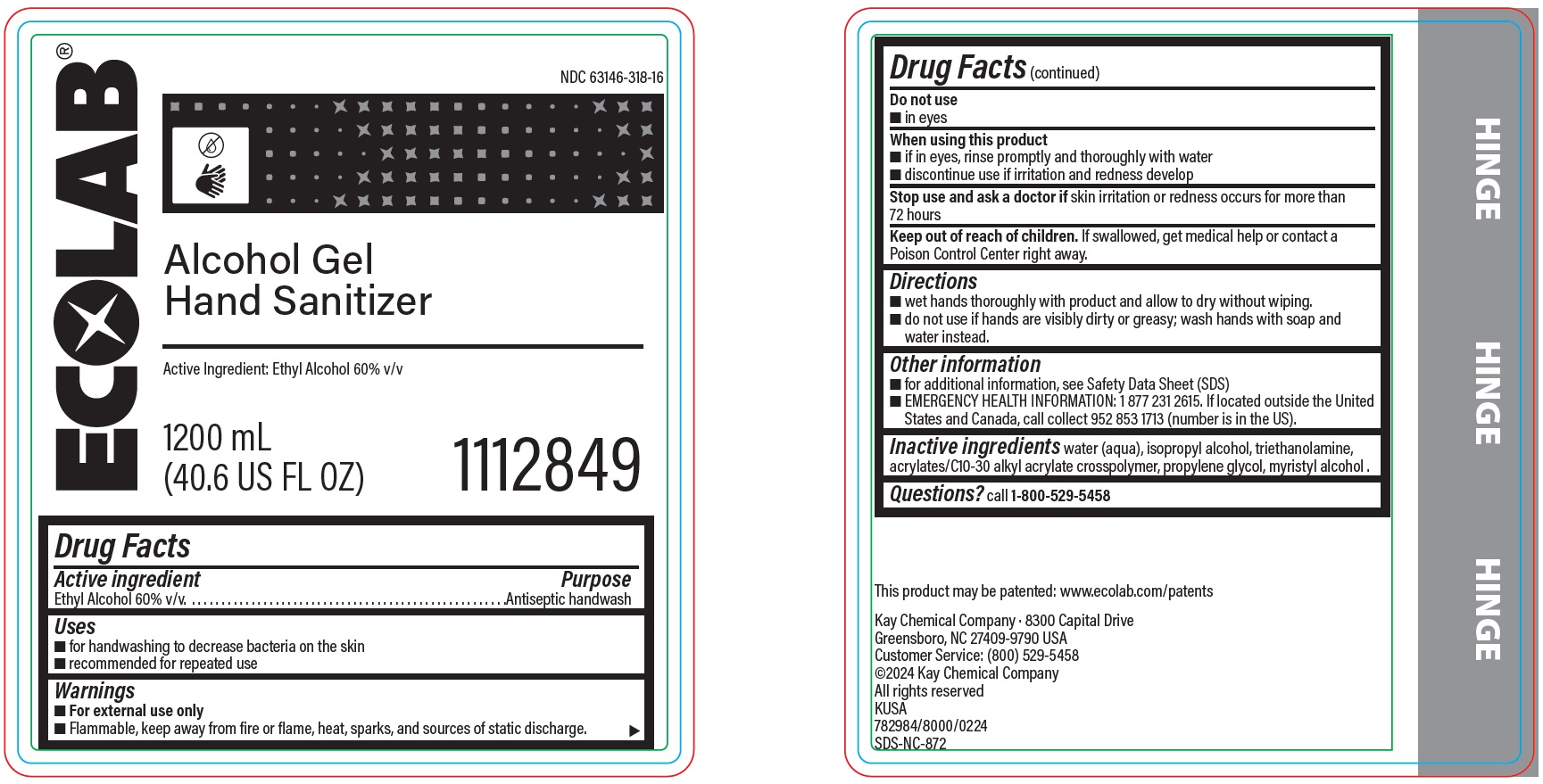
ALCOHOL GEL HAND SANITIZER- alcohol solution
Kay Chemical Company
----------
Drug Facts
Warnings
- For external use only
- Flammable, keep away from fire or flame, heat, sparks and sources of static discharge.
Directions
- wet hands thoroughly with product and allow to dry without wiping.
- do not use if hands are visibly dirty or greasy; wash hands with soap and water instead.
Other information
- For additional information, see Safety Data Sheet (SDS)
-
EMERGENCY HEALTH INFORMATION: 1 877 231 2615. If located outside the United States and Canada, call collect 952 853 1713 (number is in the US).
Inactive ingredients
water (aqua), isopropyl alcohol, triethanolamine, acrylates/C10-30 alkyl acrylate crosspolymer, propylene glycol, myristyl alcohol
Principal display panel and representative label
ECOLAB
NDC: 63146-318-16
Alcohol Gel Hand Sanitizer
Active Ingredient: Ethyl Alcohol 60% v/v
1200 mL (40.6 US FL OZ)
1112849
Kay Chemical Company · 8300 Capital Drive
Greensboro, NC 27409-9790 USA
Customer Service: (800) 529-5458
©2024 Kay Chemical Company
All rights reserved
KUSA
782984/8000/0224
SDS-NC-872
| ALCOHOL GEL HAND SANITIZER
alcohol solution |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - Kay Chemical Company (003237021) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.