CannaSerene Hot & Cold CBD Cream
CannaSerene Hot and Cold CBD Cream by
Drug Labeling and Warnings
CannaSerene Hot and Cold CBD Cream by is a Otc medication manufactured, distributed, or labeled by BioSerene Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
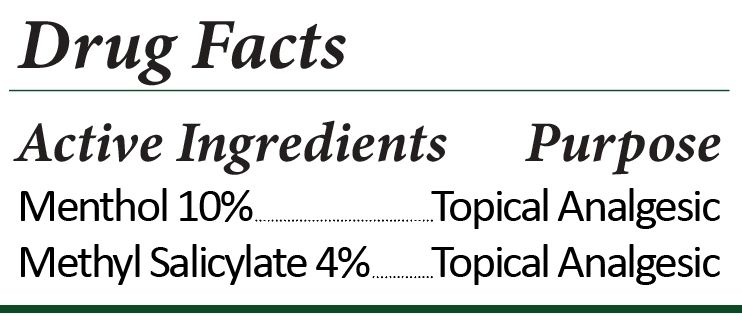
CANNASERENE HOT AND COLD CBD CREAM- hot and cold cream cream
BioSerene Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
CannaSerene Hot & Cold CBD Cream
Uses
Temporary relief from minor aches and pains of sore muscles and joints associated with: arthritis, backaches, strains, bruises, sprains, and pulled muscles.
Directions
- Adults and children 12 years of age and older: Massage over affected areas no more than 3 to 4 times daily. Wash hands after use.
- Children under 12 years of age: consult a doctor.
Warnings
FOR EXTERNAL USE ONLY.
Flammable. Keep away from fire or flame.
Allergy Alert. If prone to allergic reaction from aspirin or salicylates, consult doctor before use.
When Using This Product
- Use only as directed
- Avoid contact with eyes or mucous membranes
- Do not bandage tightly
Storage and Handling
Store at controlled room temperature 20°-25°C (68°-77°F). Keep away from direct sunlight.
Inactive Ingredients
Purified Water, Menthol, Gaultheria Procumbens (Wintergreen) Leaf Oil, Fractionated Coconut Oil, Polysorbate 80, Sorbitan Stearate, Cetearyl Alcohol, Isopropyl Myristate, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Cannabidiol, Dimethyl Isosorbide, Oenothera Biennis (EveningPrimrose) Oil, Tocopherol Acetate (VitaminE), Propylene Glycol, Diazolidinyl Urea, Iodopropynyl Butylcarbamate
| CANNASERENE HOT AND COLD CBD CREAM
hot and cold cream cream |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - BioSerene Inc. (117473724) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| BioSerene Inc. | 117473724 | manufacture(74787-1801) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.