ATRYN (antithrombin- recombinant injection, powder, lyophilized, for solution
Atryn by
Drug Labeling and Warnings
Atryn by is a Prescription medication manufactured, distributed, or labeled by Lundbeck Inc., GTC Biotherapeutics, Inc., Lonza Biologics Inc., Medimmune, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ATryn safely and effectively. See full prescribing information for ATryn.
ATryn, Antithrombin (Recombinant)
Lyophilized powder for reconstitution
Initial U.S. Approval: 2009INDICATIONS AND USAGE
ATryn is a recombinant antithrombin indicated for the prevention of peri-operative and peri-partum thromboembolic events in hereditary antithrombin deficient patients. (1)
It is not indicated for treatment of thromboembolic events in hereditary antithrombin deficient patients.
DOSAGE AND ADMINISTRATION
- For intravenous use only after reconstitution
- The dosage of ATryn is individualized for each patient. Treatment goal is to restore and maintain functional antithrombin (AT) activity levels between 80% - 120% (0.8 - 1.2 IU/mL) of normal.
- Administer loading dose as a 15-minute intravenous infusion immediately followed by continuous infusion of the maintenance dose. (2.2)
Loading Dose
(IU)Maintenance Dose
(IU/hour)Surgical Patients (100 - baseline AT activity level) x Body Weight (kg) / 2.3 (100 - baseline AT activity level) x Body Weight (kg) / 10.2 Pregnant Women (100 - baseline AT activity level) x Body Weight (kg) / 1.3 (100 - baseline AT activity level) Body Weight (kg) / 5.4 - AT activity monitoring is required for proper treatment. Check AT activity once or twice per day with dose adjustments made according to table below. (2.2)
Initial Monitor Time AT Level Dose Adjustment Recheck AT Level 2 hours after initiation of treatment < 80% Increase 30% 2 hours after each dose adjustment 80% to 120% None 6 hours after initiation of treatment or dose adjustment > 120% Decrease 30% 2 hours after each dose adjustment - Continue administration of ATryn until adequate follow-on anticoagulation has been established.
- Store at 2-8°C (36-46°F). Discard any unused portion. (2.1, 16)
DOSAGE FORMS AND STRENGTHS
- ATryn is a sterile lyophilized powder for reconstitution. Each single dose vial of ATryn contains the potency as stated on the label, which is approximately 1750 IU. (3)
CONTRAINDICATIONS
- Known hypersensitivity to goat and goat milk proteins. (4)
WARNINGS AND PRECAUTIONS
- Anaphylaxis and severe hypersensitivity reactions are possible. Should symptoms occur, treatment with the product should be discontinued, and emergency treatment should be administered. (5.1)
- The anticoagulant effect of drugs that use antithrombin to exert their anticoagulation may be altered when ATryn is added or withdrawn. To avoid excessive or insufficient anticoagulation, regularly perform coagulation tests suitable for the anticoagulant used, at close intervals, especially in the first hours following the start or withdrawal of ATryn and monitor patients for bleeding or thrombosis. (5.2)
ADVERSE REACTIONS
Most common adverse reactions reported in clinical trials at a frequency of ≥ 5% were hemorrhage and infusion site reaction. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Lundbeck Inc. at 1-800-455-1141 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
- Pregnancy Category C: Studies in pregnant women have not shown that ATryn increases the risk of fetal abnormalities if administered during the third trimester of pregnancy. No data is available for use of ATryn in earlier stages of pregnancy. (8.1)
- Labor and Delivery: ATryn is used in the treatment of peri-partum women with hereditary antithrombin deficiency. (8.2)
- Nursing Mothers: ATryn administered by infusion will be present in breast milk at estimated concentrations 1/50 to 1/100 that of concentration in blood. Use only if clearly needed. (8.3)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 6/2010
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Preparation for Administration
2.2 Recommended Dose and Schedule
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Coagulation Monitoring Tests
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Labor and Delivery
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
For Intravenous Use Only after Reconstitution
2.1 Preparation for Administration
- Bring vials to room temperature no more than 3 hours prior to reconstitution.
- Reconstitute with 10 mL Sterile Water for Injection [(WFI) not supplied with ATryn] immediately prior to use. Do not shake.
- Do not use solution containing visible particulates or if it is discolored or cloudy.
- Draw solution from one or more vials into a sterile disposable syringe for intravenous administration or add solution to an infusion bag containing 0.9% sterile sodium chloride for injection (e.g., dilute solution to obtain a concentration of 100 IU/mL).
- Administer using an infusion set with a 0.22 micron pore-size, in-line filter.
- Administer contents of infusion syringes or diluted solution within 8 to 12 hours of preparation when stored at room temperature (68-77°F (20-25°C)).
- Discard unused product in accordance with local requirements.
2.2 Recommended Dose and Schedule
- The dosage of ATryn is to be individualized based on the patient's pre-treatment functional AT activity level (expressed in percent of normal) and body weight (expressed in kilograms) and using therapeutic drug monitoring (Table 1).
- The goal of treatment is to restore and maintain functional antithrombin (AT) activity levels between 80% - 120% of normal (0.8 - 1.2 IU/mL).
- Treatment should be initiated prior to delivery or approximately 24 hours prior to surgery to ensure that the plasma antithrombin level is in the target range at that time.
- Different dosing formulae are used for the treatment of surgical and pregnant patients. Pregnant women who need a surgical procedure other than Cesarean section should be treated according to the dosing formulae for pregnant patients.
- Administer loading dose as a 15-minute intravenous infusion, immediately followed by a continuous infusion of the maintenance dose.
- AT activity monitoring and dose adjustments should be made according to Table 2.
- Continue treatment until adequate follow-on anticoagulation is established.
Table 1: Dosing Formula for Surgical Patients and Pregnant Women Loading Dose
(IU)Maintenance Dose
(IU/hour)Surgical Patients (100 - baseline AT activity level) x Body Weight (kg) / 2.3 (100 - baseline AT activity level) x Body Weight (kg) / 10.2 Pregnant Women (100 - baseline AT activity level) x Body Weight (kg) / 1.3 (100 - baseline AT activity level) Body Weight (kg) / 5.4 AT Activity Monitoring and Dose Adjustment
AT activity monitoring is required for proper treatment. Check AT activity once or twice per day with dose adjustments made according to Table 2.
Table 2: AT Activity Monitoring and Dose Adjustment Initial Monitor Time AT Level Dose Adjustment Recheck AT Level 2 hours after initiation of treatment < 80% Increase 30% 2 hours after each dose adjustment 80% to 120% None 6 hours after initiation of treatment or dose adjustment > 120% Decrease 30% 2 hours after each dose adjustment As surgery or delivery may rapidly decrease the AT activity levels, check the AT level just after surgery or delivery. If AT activity level is below 80%, an additional bolus dose may be administered to rapidly restore decreased AT activity level. In such instances, the loading dose formulae in Table 1 should be used, utilizing in the calculation the last available AT activity result. Thereafter, restart the maintenance dose at the same rate of infusion as before the bolus.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Allergic-type hypersensitivity reactions are possible. Patients must be closely monitored and carefully observed for any symptoms throughout the infusion period. Patients should be informed of the early signs of hypersensitivity reactions including hives, generalized urticaria, tightness of the chest, wheezing, hypotension, and anaphylaxis. If these symptoms occur during administration, treatment must be discontinued immediately and emergency treatment should be administered.
5.2 Coagulation Monitoring Tests
The anticoagulant effect of drugs that use antithrombin to exert their anticoagulation may be altered when ATryn is added or withdrawn. To avoid excessive or insufficient anticoagulation, coagulation tests suitable for the anticoagulant used (e.g., aPTT and anti-Factor Xa activity) are to be performed regularly, at close intervals, and in particular in the first hours following the start or withdrawal of ATryn. Additionally, monitor the patients for the occurrence of bleeding or thrombosis in such situation.
-
6 ADVERSE REACTIONS
The serious adverse reaction that has been reported in clinical studies is hemorrhage (intra-abdominal, hemarthrosis and post procedural). The most common adverse events reported in clinical trials at a frequency of ≥ 5% are hemorrhage and infusion site reaction.
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse reactions that occurred in clinical trials with hereditary AT deficient patients are shown in Table 3 by System Organ Class.
Table 3: Adverse Reactions in Hereditary AT Deficient Patients (one event per patient, 2% of total population, n=47) Gastrointestinal Disorders Intra-abdominal Hemorrhage General Disorders and Administration Site Disorders Application Site Pruritus Feeling Hot Non-cardiac Chest Pain Investigations Hepatic Enzyme Abnormal Musculoskeletal and Connective Tissue Disorders Hemarthrosis Renal and Urinary Disorders Hematuria Vascular Disorders Hematoma Immunogenicity
For ATryn, a potential safety issue is the development of an immunological reaction to the recombinant protein or any of the potential contaminating proteins. Assays were developed and used to detect antibodies directed against antithrombin (Recombinant), goat AT, or goat-milk proteins. No confirmed specific immunological reaction was seen in any of the patients tested, nor were there any clinical adverse events that might indicate such a response.
A post-marketing patient registry has been established to collect additional data on the immunogenic potential of ATryn in patients treated with ATryn on more than one occasion. Physicians are encouraged to participate in the registry by collecting pre- and post-treatment serum samples from patients according to instructions provided by Lundbeck Inc. and submitting them to Lundbeck Inc. for analysis for the development of antibodies to antithrombin (Recombinant). Serum samples should be collected within one week before initiation of treatment and on days 1, 7 and 28 days from initiation of treatment. Physicians wanting to participate in this program are encouraged to contact Lundbeck Inc. at 1-800-455-1141. Lundbeck Inc. will provide detailed instructions for the collection, processing and shipping of samples, as well as all tubes and labels that are necessary for the collection and processing of samples.
-
7 DRUG INTERACTIONS
The anticoagulant effect of heparin and low molecular weight heparin (LMWH) is enhanced by antithrombin. The half-life of antithrombin may be altered by concomitant treatment with these anticoagulants due to an altered antithrombin turnover. Thus, concurrent administration of antithrombin with heparin, low molecular weight heparin, or other anticoagulants that use antithrombin to exert their anticoagulant effect must be monitored clinically and biologically. To avoid excessive anticoagulation, regular coagulation tests (aPTT, and where appropriate, anti-Factor Xa activity) are to be performed at close intervals, with adjustment in dosage of the anticoagulant as necessary.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C: In rats, a dose of 210 mg/kg/day ATryn (5-6 times the human dose for pregnant women) administered during most of the pregnancy and entire lactation showed a slight but statistically significant increase in pup mortality in day one through day four when compared to concurrent control (90% compared to 94% viability index for 210 mg/kg/day versus control). This slight statistical difference does not reflect a true treatment-related effect. This same dose was shown to be safe in a second rat study when administered around parturition and during lactation where the no adverse effect level for dam and pups was 210 mg/kg/day.
There are no adequate and well-controlled studies in pregnant women. Because animal reproductive studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Studies in pregnant women have not shown that ATryn increases the risk of fetal abnormalities if administered during the third trimester of pregnancy. In clinical trials in hereditary AT deficient patients, 22 pregnant women have been treated with ATryn around parturition.
No adverse reactions were reported in 22 neonates born from pregnant women treated with ATryn during clinical trials.
8.2 Labor and Delivery
ATryn is indicated for the treatment of pregnant women during the peri-partum period. Pregnant patients who need a surgical procedure other than Cesarean section are to be treated according to the dosing formulae for pregnant patients.
8.3 Nursing Mothers
ATryn will be present in breast milk at levels estimated to be 1/50 to 1/100 of its concentration in the blood. This level is the same as that estimated to be present in breast milk of normal lactating women which is not known to be harmful to breastfed neonates. However, caution should be exercised when ATryn is administered to a nursing woman. Use only if clearly needed.
In 2 reproductive toxicology studies performed in rats, antithrombin (Recombinant) was administered to pregnant dams at doses up to 210 mg/kg/day, resulting in supraphysiologic plasma levels of antithrombin. Pups were allowed to breastfeed and were monitored for changes in prothrombin (PT) or aPTT, as well as pup viability, body weight at birth, growth, and development. In these studies, there were no adverse effects in offspring who consumed milk from dams treated with ATryn.
8.5 Geriatric Use
Clinical studies of ATryn did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
11 DESCRIPTION
ATryn for Injection is a nanofiltered, sterile, terminally heat treated, lyophilized dosage form. Antithrombin (Recombinant), active ingredient of ATryn, is a recombinant human antithrombin. It is a 432 amino acid glycoprotein with a molecular weight of approximately 57,215 Daltons. The molecular formula is: C2191H3457N583O656S18. Antithrombin (Recombinant) is produced by recombinant DNA technology using genetically engineered goats into which the DNA coding sequence for human antithrombin has been introduced along with a mammary gland specific DNA sequence, which directs the expression of the antithrombin into the milk. The goats in which antithrombin (Recombinant) is produced are USDA certified scrapie-free, and controlled for specific pathogens.
The amino acid sequence of Antithrombin (Recombinant) is identical to that of human plasma-derived antithrombin. Antithrombin (Recombinant) and plasma-derived antithrombin both contain six cysteine residues forming three disulphide bridges and 3-4 N-linked carbohydrate moieties. The glycosylation profile of Antithrombin (Recombinant) is different from plasma-derived antithrombin, which results in an increased heparin affinity. When assayed in the presence of excess of heparin the potency of the recombinant product is not different from that of plasma-derived product.
Each vial of ATryn is tested for potency stated on the product label using a reference standard calibrated against the World Health Organization international standard for antithrombin concentrate. In addition to Antithrombin (Recombinant), each vial of the product contains 100 mg glycine, 79 mg sodium chloride, and 26 mg sodium citrate. When reconstituted with 10 mL Sterile Water for Injection, the pH is approximately 7.0. Following reconstitution, the solution may be further diluted into 0.9% sodium chloride for injection.
ATryn does not contain any preservatives nor is it formulated with human plasma proteins. Antithrombin (Recombinant) is affinity purified using a heparin immobilized resin and contains no detectable heparin (<0.0002 IU heparin per IU antithrombin) in the final product.
The purification and drug product manufacturing processes have been validated to demonstrate its capacity for removal and/or inactivation of viruses4. Results of removal and/or inactivation for each of the steps are shown in Table 4.
Table 4: Viral Clearance Results (log10 reductions) Process Step Pseudorabies Virus Xenotropic Murine Retrovirus Human Adenovirus Porcine Parvovirus NA = Not Applicable since log10 reduction was less than 1.0. Tangential Flow Filtration ≥5.1 Affinity Chromatography 1.6 1.2 NA 1.4 Nanofiltration ≥3.8 ≥6.3 ≥3.7 Ion Exchange Chromatography 3.6 1.0 ≥7.1 NA Hydrophobic Interaction Chromatography ≥5.6 ≥4.4 ≥4.8 ≥5.7 Heat Treatment 2.8 ≥5.0 ≥1.8 2.4 Total Reduction ≥18.7 ≥15.4 ≥20.0 ≥13.2 In addition, although the goats are from a closed, USDA certified scrapie-free herd, the purification process was challenged to remove prions. The manufacturing steps were shown capable of achieving the following log10 reductions: 2.0 (tangential filtration), 2.2 (affinity column), ≥ 3.3 (ion exchange column), ≥ 3.8 (hydrophobic interaction column).
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Antithrombin (AT) plays a central role in the regulation of hemostasis. AT is the principal inhibitor of thrombin and Factor Xa5, the serine proteases that play pivotal roles in blood coagulation. AT neutralizes the activity of thrombin and Factor Xa by forming a complex which is rapidly removed from the circulation. The ability of antithrombin to inhibit thrombin and Factor Xa can be enhanced by greater than 300 to 1000 fold when AT is bound to heparin.
12.2 Pharmacodynamics
Hereditary AT deficiency causes an increased risk of venous thromboembolism (VTE). During high-risk situations such as surgery or trauma or for pregnant women, during the peri-partum period, the risk of development of VTEs as compared to the normal population in these situations is increased by a factor 10 to 506,7.
In hereditary antithrombin deficient patients ATryn restores (normalizes) plasma AT activity levels during peri-operative and peri-partum periods.
12.3 Pharmacokinetics
In an open-label, single dose pharmacokinetic study, male and female patients (≥ 18 years of age) with hereditary AT deficiency, received either 50 (n = 9, all females) or 100 (n = 6, 2 males and 4 females) IU/kg ATryn intravenously. These patients were not in high-risk situations. The baseline corrected pharmacokinetic parameters for antithrombin (Recombinant) are summarized in Table 5.
Table 5: Baseline Corrected Mean Pharmacokinetic Parameters (%CV) Parameter 50 IU/kg 100 IU/kg CL (mL/hr/kg) 9.6 (34.4) 7.2 (15.3) Half-life (hrs) 11.6 (84.7) 17.7 (60.9) MRT (hrs) 16.2 (74.9) 20.5 (40.2) Vss (mL/kg) 126.2 (37.4) 156.1 (43.4) Incremental recovery [mean (%CV)] was 2.24 (20.2) and 1.94 (14.8) %/IU/kg body weight for 50 and 100 IU/kg, respectively.
Population pharmacokinetic analysis of hereditary deficient patients in a high risk situation revealed that the clearance and volume of distribution in pregnant patients were (1.38 L/h and 14.3 L respectively) which are higher than non-pregnant patients (0.67 L/h and 7.7 L respectively). Therefore, distinct dosing formulae for surgical and pregnant patients should be used (see 2.2, Recommended Dose and Schedule).
As compared to plasma derived antithrombin, ATryn has a shorter half-life and more rapid clearance (approximately nine and seven times, respectively).
Pharmacokinetics may be influenced by concomitant heparin administration, as well as surgical procedures, delivery, or bleeding. AT activity monitoring (see 2.2, Recommended Dose and Schedule) should be performed to properly treat such patients.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility
Carcinogenesis: No carcinogenicity data for ATryn are available in animals or humans.
Mutagenesis and Genotoxicity: ATryn was not mutagenic when tested in the Ames bacterial test and in in vitro cytogenetic assays nor was it shown to be genotoxic when tested in an in vivo test to assess chromosomal aberration.
Impairment of Fertility: No studies have been conducted to evaluate the effects of ATryn on fertility in humans.
13.2 Animal Toxicology and/or Pharmacology
Pharmacokinetic and toxicokinetic (1 single, 2 repeated dose) studies of antithrombin (Recombinant) were performed in mice, rats, dogs and monkeys. In toxicokinetic studies in monkeys the area under the curve was 3-4 times greater than in the rat at all doses used.
The toxicological profile of antithrombin (Recombinant) administered by the intravenous route as bolus injections and infusions has been evaluated in both single- and repeat-dose studies performed in rats, dogs, and monkeys across a range of doses from 2.1 to 360 mg/kg. The highest doses in the single dose toxicity studies in rats and dogs were 360 mg/kg and 210 mg/kg, respectively. Toxicities observed were limited to transient injection site swelling observed in rats and dogs at the highest doses tested, and increased AST at highest dose in the dog study, both resolved during recovery period.
The highest dose in the 28-day repeated-dose toxicity study in rats was 360 mg/kg/day. The toxicity at this dose was limited to transient limb swelling and local injection site bruising and swelling. The highest dose in the 14-day repeated-dose toxicity study in monkeys was 300 mg/kg/day or approximately 7-8 times human dose. Toxicities observed in female monkeys at this dose included internal bleeding, hematological changes and liver toxicity, with one out of three female animals showing multifocal hepatic necrosis. Both sexes showed increased AST and ALK on day 15, with both parameters returning to normal by day 22. There was no adverse effect in monkeys dosed with 120 mg/kg/day.
-
14 CLINICAL STUDIES
The efficacy of ATryn to prevent the occurrence of venous thromboembolic events was assessed by comparing the incidence of the occurrence of such events in 31 ATryn treated hereditary AT deficient patients with the incidence in 35 human plasma-derived AT treated hereditary AT deficient patients. Data on ATryn-treated patients were derived from two prospective, single-arm, open-label studies. Data on plasma AT treated patients were collected from a prospectively designed concurrently conducted retrospective chart review. Patients in both studies had confirmed hereditary AT deficiency (AT activity ≤ 60% of normal) and a personal history of thromboembolic events. Patients had to be treated in the peri-operative and peri-partum period. ATryn was administered as a continuous infusion for at least 3 days, starting one day prior to the surgery or delivery. Plasma AT was administered for at least two days as single bolus infusions. Due to the retrospective nature of the study, dosing was done with the locally available AT concentrate according to the local practice.
The occurrence of a venous thromboembolic event was confirmed if signs and symptoms for such events were confirmed by a specific diagnostic assessment, or when treatment for an event was initiated based on diagnostic imaging, without the presence of signs and symptoms. The efficacy was assessed during treatment with AT and up to 7 days after stopping AT treatment.
In the ATryn-treated group there was one confirmed diagnosis of an acute deep vein thrombosis (DVT). The incidence of any thromboembolic event from the start of treatment to 7 days after last dosing is summarized by treatment group in Table 6 as are the Clopper-Pearson exact 95% CI for the proportion of patients with a thromboembolic event and the exact 95% lower confidence bound for the difference between treatments.
Table 6: Overall Incidence of Any Confirmed Thromboembolic Event Plasma AT ATryn No. of Pts. Assessed No. of Pts. With Events % of Pts. With Events 95% CI* No. of Pts. Assessed No. of Pts. With Events % of Pts. With Events 95% CI Lower 95% Confidence Bound of Difference The 95% confidence intervals were calculated using Clopper-Pearson methodology. AT=Antithrombin; No.=Number; Pts.=Patients; CI=Confidence Interval 35 0 0.0 0.00,
10.0031 1 3.2 0.08,
16.70-0.167 The lower 95% confidence bound of difference between treatment groups was -0.167, a value that is greater than the pre-specified lower confidence bound of -0.20. This demonstrates that ATryn was non-inferior to plasma AT in terms of the prevention of peri-operative or peri-partum thromboembolic events.
Supportive data come from a study in the same population with 5 hereditary AT deficient patients treated on 6 occasions in a compassionate use program and provides additional reassurance of the efficacy of ATryn. None of these patients reported a thromboembolic event.2
-
15 REFERENCES
- Patnaik MM, Moll S. Inherited antithrombin deficiency: a review. Haemophilia 2008;14:1229-39.
- Konkle BA, Bauer KA, Weinstein R, Greist A, Holmes HE, Bonfiglio J. Use of recombinant human antithrombin in patients with congenital antithrombin deficiency undergoing surgical procedures. Transfusion 2003 March;43(3):390-4.
- Edmunds T, Van Patten SM, Pollock J et al. Transgenically produced human antithrombin: structural and functional comparison to human plasma-derived antithrombin. Blood 1998 June 15;91(12):4561-71.
- Echelard Y, Meade H, Ziomek C. The first biopharmaceutical from transgenic animals: ATryn. Modern Biopharmaceuticals 2005;995-1016.
- Maclean PS, Tait RC. Hereditary and acquired antithrombin deficiency: epidemiology, pathogenesis and treatment options. Drugs 2007;67(10):1429-40.
- Buchanan GS, Rodgers GM, Ware Branch. The inherited thrombophilias: genetics, epidemiology, and laboratory evaluation. Best Pract Res Clin Obstet Gynaecol 2003 June;17(3):397-411.
- Walker ID, Greaves M, Preston FE. Investigation and management of heritable thrombophilia. Br J Haematol 2001;114:512-28.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Dosage Form
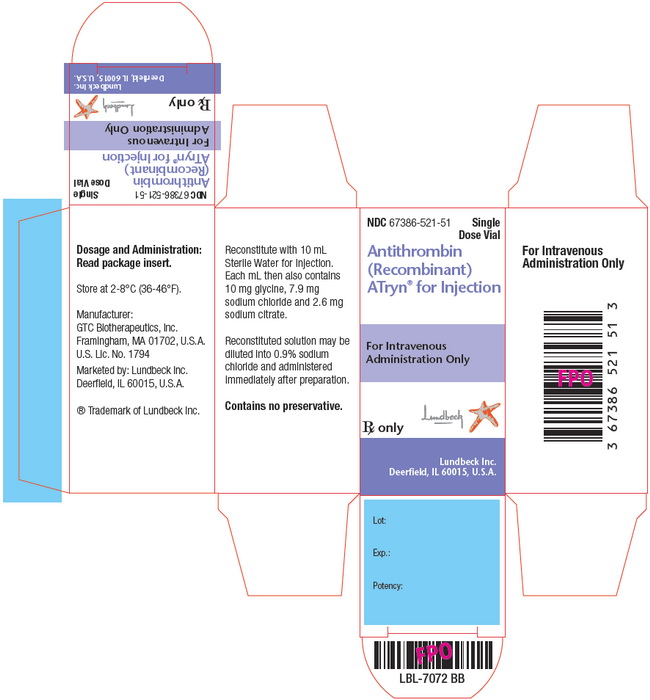
NDC: 67386-521-51
Approximately 1750 IU/vial in a sterile white to off-white lyophilized powder for reconstitution. Each carton contains one single dose vial of ATryn.
The actual potency of ATryn is stated on the vial label and carton.
Storage and Handling
Store ATryn refrigerated at between 2-8°C (36-46°F).
Do not use product beyond the expiration date printed on the package. Discard unused portions.
-
17 PATIENT COUNSELING INFORMATION
Inform patients that allergic-type hypersensitivity reactions are possible and instruct them to inform their physicians about any past or present known hypersensitivity to goats or goat milk proteins prior to treatment with ATryn. Inform patients of the early signs of hypersensitivity reactions including hives, generalized urticaria, tightness of the chest, wheezing, hypotension, and anaphylaxis and to notify their health care provider immediately if these events develop.
Inform patients about the risk of bleeding when ATryn is administered with other anticoagulants and instruct them to notify their physicians of any bleeding events while on treatment with ATryn.
Manufacturer:
GTC Biotherapeutics, Inc.
Framingham, MA 01702, U.S.A.
U.S. License No. 1794Marketed by:
Lundbeck Inc.
Deerfield, IL 60015, U.S.A.® Trademark of Lundbeck Inc.
Revised: April 2009
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ATRYN
antithrombin (recombinant) injection, powder, lyophilized, for solutionProduct Information Product Type Item Code (Source) NDC: 67386-521 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANTITHROMBIN ALFA (UNII: AWV6I5L6H2) (ANTITHROMBIN ALFA - UNII:AWV6I5L6H2) ANTITHROMBIN ALFA 1750 [iU] in 10 mL Inactive Ingredients Ingredient Name Strength GLYCINE (UNII: TE7660XO1C) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM CITRATE (UNII: 1Q73Q2JULR) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 67386-521-51 1 in 1 CARTON 1 10 mL in 1 VIAL Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125284 02/06/2009 Labeler - Lundbeck Inc. (018343595) Establishment Name Address ID/FEI Business Operations GTC Biotherapeutics, Inc. 807934260 ANALYSIS Establishment Name Address ID/FEI Business Operations Lonza Biologics Inc. 093149750 MANUFACTURE Establishment Name Address ID/FEI Business Operations Medimmune, LLC 190639906 MANUFACTURE
Trademark Results [Atryn]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ATRYN 76184729 2743233 Live/Registered |
REVO BIOLOGICS, INC. 2000-12-20 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.