X-RAY by Washington Homeopathic Products X-RAY X
X-RAY by
Drug Labeling and Warnings
X-RAY by is a Homeopathic medication manufactured, distributed, or labeled by Washington Homeopathic Products. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
X-RAY- lactose, x-ray exposed- 1000 rad pellet
Washington Homeopathic Products
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
X-RAY X
STOP USE AND ASK DOCTOR
If symptoms persist/worsen or if pregnant/nursing, consult your practitioner.
DIRECTIONS
Adults: 4 drops into a tsp. of water 3 times a day. Children: 1/2 dose. Repeat at greater intervals as condition subsides. Or as directed bya lic. practitioner.
PRINCIPAL DISPLAY PANEL
The OTC potency range of ALFALFA is 2x–30x, 1c–30c, 200c, 1m, 10m, 50m, and CM.

Availability is subject to change.
All WHP single remedies are made to order; thus, the labels are printed on the same label stock, as the orders are filled.
‘Bottle Size,’ ‘Potency,’ and ‘Alcohol Percentage’ vary on the label depending on customer choice.
Standard bottle sizes for dilution-form remedies are 15ml, 30ml, 50ml, and 100ml.
| X-RAY
lactose, x-ray exposed- 1000 rad pellet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
 |
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Washington Homeopathic Products (084929389) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Washington Homeopathic Products | 084929389 | manufacture(71919-756) | |
Trademark Results [X-RAY]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 X-RAY 98682497 not registered Live/Pending |
dbrand inc. 2024-08-05 |
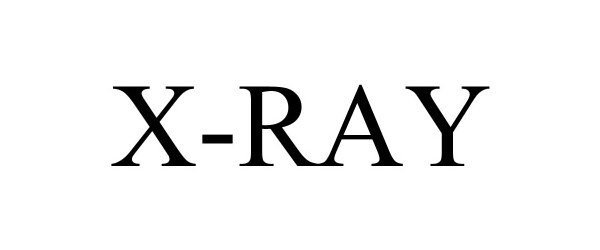 X-RAY 98421925 not registered Live/Pending |
Dr. Z Amps, Inc. 2024-02-26 |
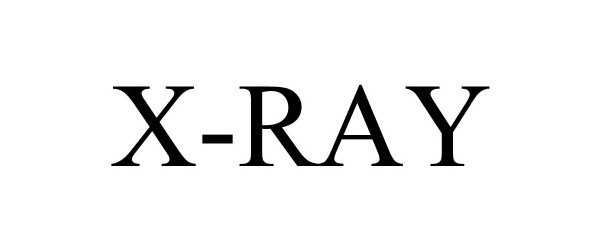 X-RAY 98141886 not registered Live/Pending |
Tangibly Inc. 2023-08-21 |
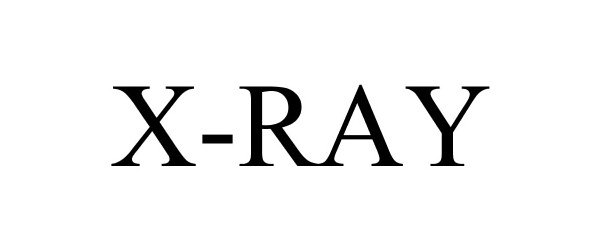 X-RAY 87913642 not registered Live/Pending |
PARSONS XTREME GOLF, LLC 2018-05-09 |
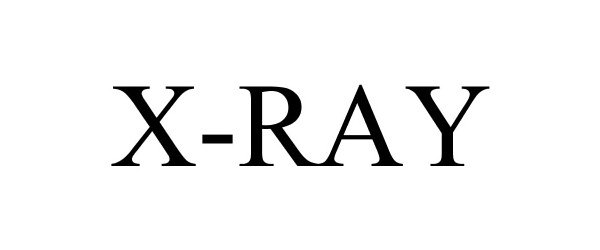 X-RAY 86491709 5041924 Live/Registered |
SIG SAUER Inc. 2014-12-29 |
 X-RAY 86479655 4907211 Live/Registered |
Rescuecom Corporation 2014-12-12 |
 X-RAY 85433980 4488866 Live/Registered |
Amazon Technologies, Inc. 2011-09-28 |
 X-RAY 85192014 not registered Dead/Abandoned |
Carmichael, Martin M 2010-12-07 |
 X-RAY 85108928 4502017 Live/Registered |
Sports Outdoor And Recreation (SOAR) Park 2010-08-17 |
 X-RAY 79053830 4085637 Dead/Cancelled |
RUOTEMILANO S.r.l. 2008-04-07 |
 X-RAY 77478352 not registered Dead/Abandoned |
Estes-Cox Corp. 2008-05-19 |
 X-RAY 77195696 3541065 Dead/Cancelled |
Carmichael, Martin M 2007-06-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.