EXTRANEAL- icodextrin, sodium chloride, sodium lactate, calcium chloride, magnesium chloride injection, solution
EXTRANEAL by
Drug Labeling and Warnings
EXTRANEAL by is a Prescription medication manufactured, distributed, or labeled by Vantive US Healthcare LLC, Baxter Healthcare S.A.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Health Care Provider Letter
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
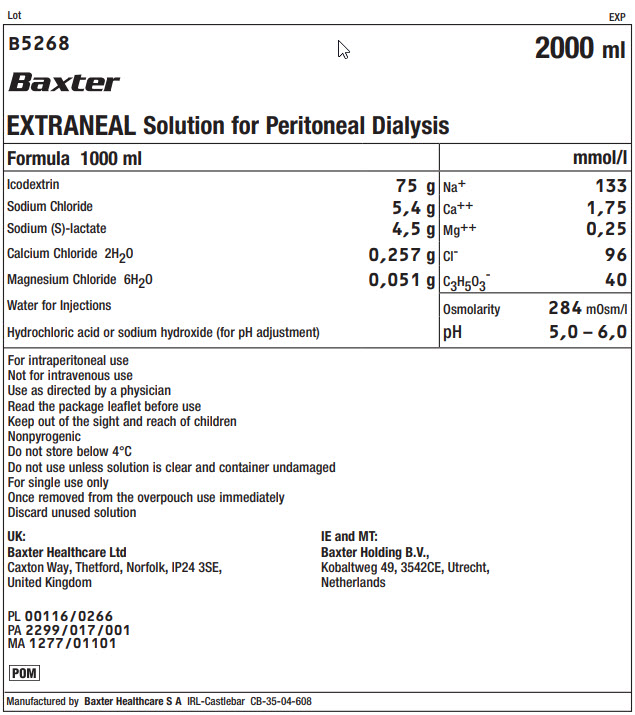
Lot EXP
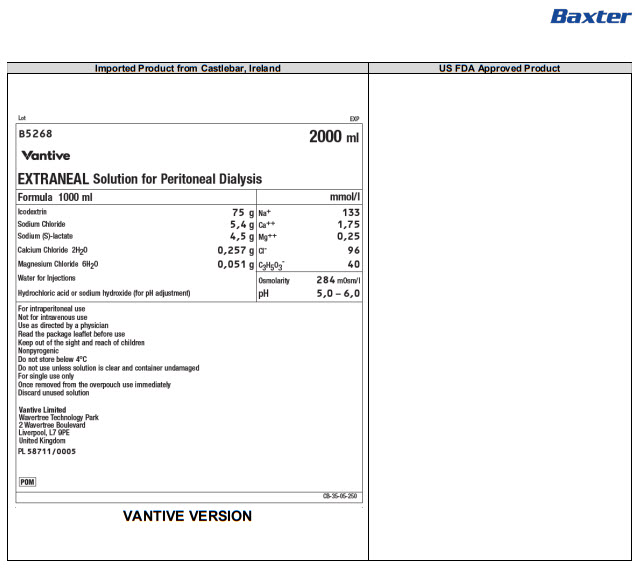
B5268 2000 ml
Baxter
EXTRANEAL Solution for Peritoneal Dialysis
Formula 1000 ml
mmol/l
Icodextrin
75 g
Na+
133
Sodium Chloride
5,4 g
Ca++
1,75
Sodium (S)-lactate
4,5 g
Mg++
0,25
Calcium Chloride 2H 20
0,257 g
Clˉ
96
Magnesium Chloride 6H 20
0,051 g
C 3H 50 3ˉ
40
Water for Injections
Osmolarity
284mOsm/l
Hydrochloric acid or sodium hydroxide (for pH adjustment)
pH
5,0 – 6,0
For intraperitoneal use
Not for intravenous use
Use as directed by a physician
Read the package leaflet before use
Keep out of the sight and reach of children
Nonpyrogenic
Do not store below 4°C
Do not use unless solution is clear and container undamaged
For single use only
Once removed from the overpouch use immediately
Discard unused solutionUK:
Baxter Healthcare Ltd
Caxton Way, Thetford, Norfolk, IP24 3SE,
United KingdomIE and MT:
Baxter Holding B.V.,
Kobaltweg 49, 3542CE, Utrecht,
NetherlandsPL 00116/0266
PA 2299/017/001
MA 1277/01101POM Symbol
Manufactured by Baxter Healthcare S A IRL-Castlebar CB-35-04-608
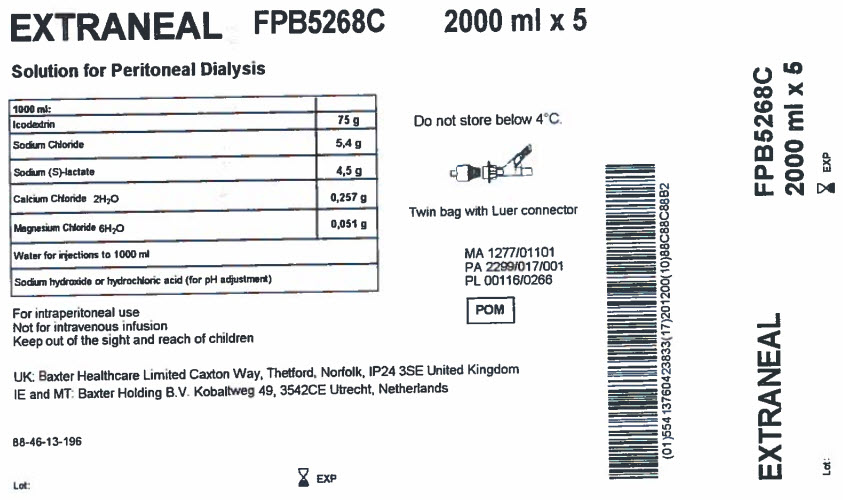
EXTRANEAL
FPB5268C
2000 ml x 5Solution for Peritoneal Dialysis
1000 ml
Icodextrin
75 g
Sodium Chloride
5,4 g
Sodium (S)-lactate
4,5 g
Calcium Chloride 2H 20
0,257 g
Magnesium Chloride 6H 20
0,051 g
Water for injections to 1000 ml
Sodium hydroxide or hydrochloric acid (for pH adjustment)
For intraperitoneal use
Not for intravenous infusion
Keep out of the sight and reach of childrenUK: Baxter Healthcare Limited Caxton Way, Thetford, Norfolk, IP24 3SE United Kingdom
IE and MT: Baxter Holding B.V. Kobaltweg 49, 3542CE, Utrecht, Netherlands88-46-13-196
Lot: EXP
Do not store below 4°C
Twin bag with Luer connector
MA 1277/01101
PA 2299/017/001
PL 00116/0266POM Symbol
Barcode
(01)554 13760423833(17)201200(10)88C88C88B2EXTRANEAL
FPB5268C
2000 ml x 5Lot: EXP
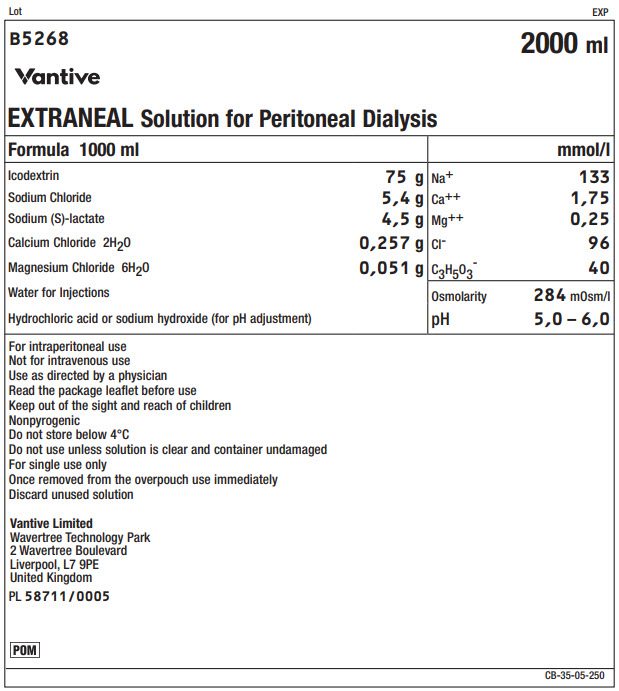
Lot EXP
B5268 2000 ml
Vantive
EXTRANEAL Solution for Peritoneal Dialysis
Formula 1000 ml
mmol/l
Icodextrin
75 g
Na+
133
Sodium Chloride
5,4 g
Ca++
1,75
Sodium (S)-lactate
4,5 g
Mg++
0,25
Calcium Chloride 2H 20
0,257 g
Clˉ
96
Magnesium Chloride 6H 20
0,051 g
C 3H 50 3ˉ
40
Water for Injections
Osmolarity
284mOsm/l
Hydrochloric acid or sodium hydroxide (for pH adjustment)
pH
5,0 – 6,0
For intraperitoneal use
Not for intravenous use
Use as directed by a physician
Read the package leaflet before use
Keep out of the sight and reach of children
Nonpyrogenic
Do not store below 4°C
Do not use unless solution is clear and container undamaged
For single use only
Once removed from the overpouch use immediately
Discard unused solutionVantive Limited
Wavertree Technology Park
2 Wavertree Boulevard
Liverpool, L7 9PE
United KingdomPL 58711/0005
POM Symbol
CB-35-05-250
EXTRANEAL
FPB5268C
2000 ml x 5Solution for Peritoneal Dialysis
1000 ml
Icodextrin
75 g
Sodium Chloride
5,4 g
Sodium (S)-lactate
4,5 g
Calcium Chloride 2H 20
0,257 g
Magnesium Chloride 6H 20
0,051 g
Water for injections to 1000 ml
Sodium hydroxide or hydrochloric acid (for pH adjustment)
For intraperitoneal use
Not for intravenous infusion
Keep out of the sight and reach of childrenVantive Limited Wavertree Technology Park 2 Wavertree Boulevard Liverpool, L7 9PE United Kingdom
88-46-14-007
Lot: EXP
UK Only
Do not store below 4°C
Twin bag with Luer connector
PL 58711/0005
POM Symbol
Barcode
(01)57332414203103 (17)201200(10)88C88C88B2EXTRANEAL
FPB5268C
2000 ml x 5Lot: EXP
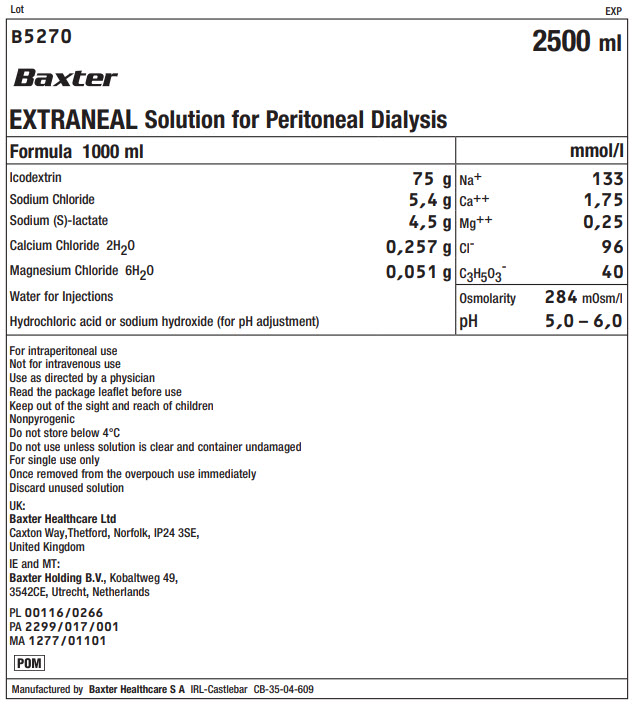
Lot EXP
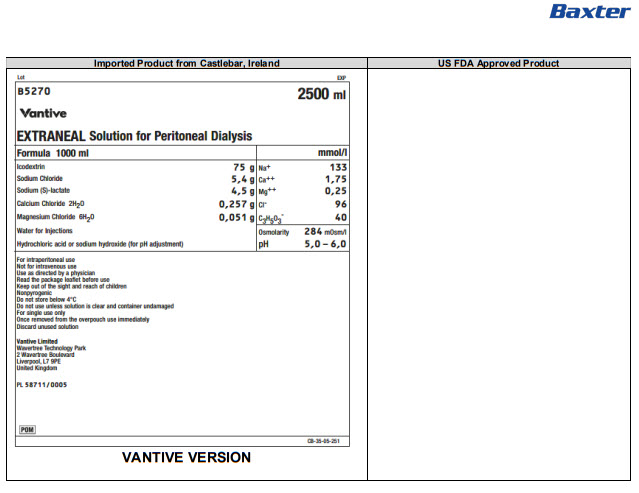
B5270 2500 ml
Baxter
EXTRANEAL Solution for Peritoneal Dialysis
Formula 1000 ml
mmol/l
Icodextrin
75 g
Na+
133
Sodium Chloride
5,4 g
Ca++
1,75
Sodium (S)-lactate
4,5 g
Mg++
0,25
Calcium Chloride 2H 20
0,257 g
Clˉ
96
Magnesium Chloride 6H 20
0,051 g
C 3H 50 3ˉ
40
Water for Injections
Osmolarity
284mOsm/l
Hydrochloric acid or sodium hydroxide (for pH adjustment)
pH
5,0 – 6,0
For intraperitoneal use
Not for intravenous use
Use as directed by a physician
Read the package leaflet before use
Keep out of the sight and reach of children
Nonpyrogenic
Do not store below 4°C
Do not use unless solution is clear and container undamaged
For single use only
Once removed from the overpouch use immediately
Discard unused solutionUK:
Baxter Healthcare Ltd
Caxton Way, Thetford, Norfolk, IP24 3SE,
United KingdomIE and MT:
Baxter Holding B.V., Kobaltweg 49,
3542CE, Utrecht, NetherlandsPL 00116/0266
PA 2299/017/001
MA 1277/01101POM Symbol
Manufactured by Baxter Healthcare S A IRL-Castlebar CB-35-04-609
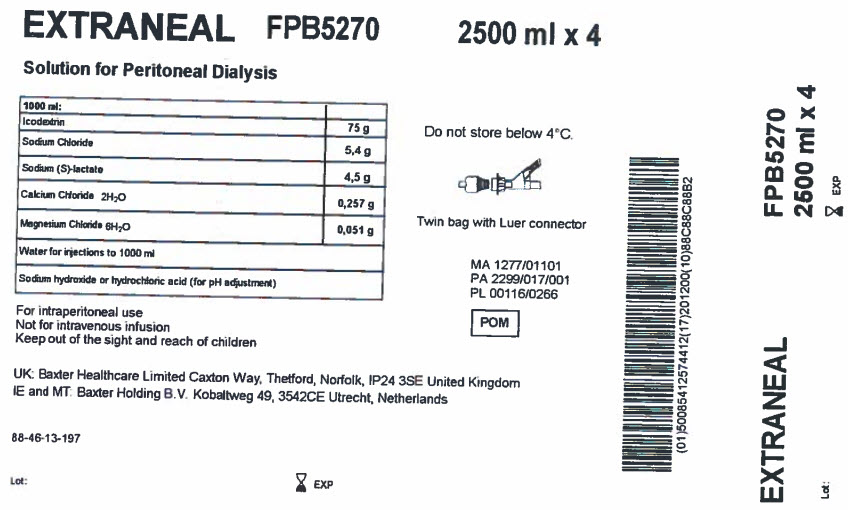
EXTRANEAL
FPB5270
2500 ml x 4Solution for Peritoneal Dialysis
1000 ml
Icodextrin
75 g
Sodium Chloride
5,4 g
Sodium (S)-lactate
4,5 g
Calcium Chloride 2H 20
0,257 g
Magnesium Chloride 6H 20
0,051 g
Water for injections to 1000 ml
Sodium hydroxide or hydrochloric acid (for pH adjustment)
For intraperitoneal use
Not for intravenous infusion
Keep out of the sight and reach of childrenUK: Baxter Healthcare Limited Caxton Way, Thetford, Norfolk, IP24 3SE United Kingdom
IE and MT: Baxter Holding B.V. Kobaltweg 49, 3542CE, Utrecht, Netherlands88-46-13-197
Lot: EXP
Do not store below 4°C
Twin bag with Luer connector
MA 1277/01101
PA 2299/017/001
PL 00116/0266POM Symbol
Barcode
(01)50085412574412(17)201200(10)88C88C88B2EXTRANEAL
FPB5270
2000 ml x 4Lot: EXP
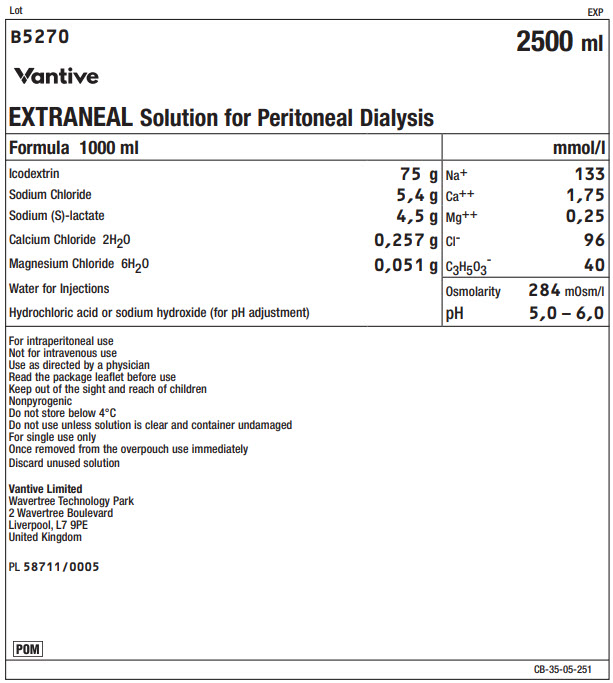
Lot EXP
B5270 2500 ml
Vantive
EXTRANEAL Solution for Peritoneal Dialysis
Formula 1000 ml
mmol/l
Icodextrin
75 g
Na+
133
Sodium Chloride
5,4 g
Ca++
1,75
Sodium (S)-lactate
4,5 g
Mg++
0,25
Calcium Chloride 2H 20
0,257 g
Clˉ
96
Magnesium Chloride 6H 20
0,051 g
C 3H 50 3ˉ
40
Water for Injections
Osmolarity
284mOsm/l
Hydrochloric acid or sodium hydroxide (for pH adjustment)
pH
5,0 – 6,0
For intraperitoneal use
Not for intravenous use
Use as directed by a physician
Read the package leaflet before use
Keep out of the sight and reach of children
Nonpyrogenic
Do not store below 4°C
Do not use unless solution is clear and container undamaged
For single use only
Once removed from the overpouch use immediately
Discard unused solutionVantive Limited
Wavertree Technology Park
2 Wavertree Boulevard
Liverpool, L7 9PE
United KingdomPL 58711/0005
POM Symbol
CB-35-05-251
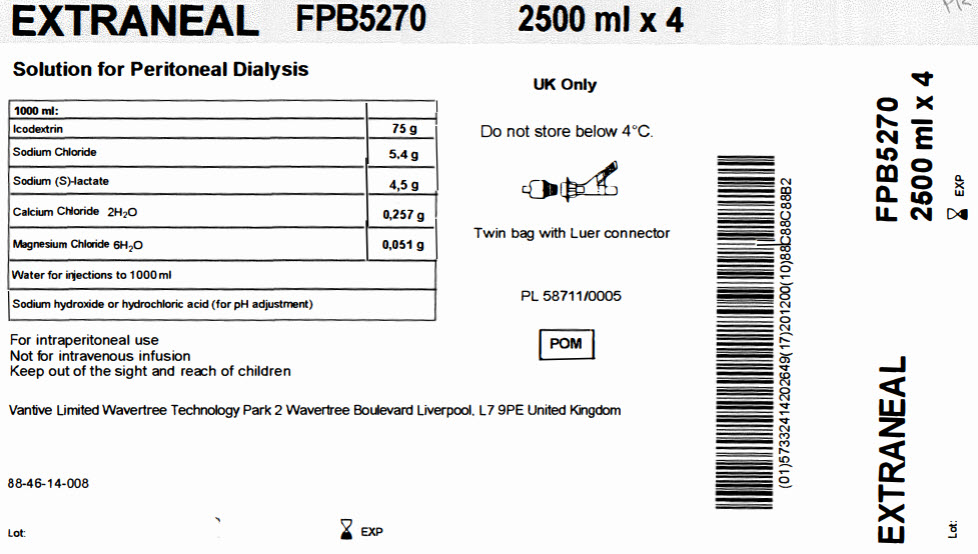
EXTRANEAL
FPB5270
2500 ml x 4Solution for Peritoneal Dialysis
1000 ml
Icodextrin
75 g
Sodium Chloride
5,4 g
Sodium (S)-lactate
4,5 g
Calcium Chloride 2H 20
0,257 g
Magnesium Chloride 6H 20
0,051 g
Water for injections to 1000 ml
Sodium hydroxide or hydrochloric acid (for pH adjustment)
For intraperitoneal use
Not for intravenous infusion
Keep out of the sight and reach of childrenVantive Limited Wavertree Technology Park 2 Wavertree Boulevard Liverpool, L7 9PE United Kingdom
88-46-14-008
Lot: EXP
UK Only
Do not store below 4°C
Twin bag with Luer connector
PL 58711/0005
POM Symbol
Barcode
(01)57332414202649 (17)201200(10)88C88C88B2EXTRANEAL
FPB52702500 ml x 4
Lot: EXP
-
INGREDIENTS AND APPEARANCE
EXTRANEAL
icodextrin, sodium chloride, sodium lactate, calcium chloride, magnesium chloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0941-0681 Route of Administration INTRAPERITONEAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ICODEXTRIN (UNII: 2NX48Z0A9G) (ICODEXTRIN - UNII:2NX48Z0A9G) ICODEXTRIN 7.5 g in 100 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 540 mg in 100 mL SODIUM LACTATE (UNII: TU7HW0W0QT) (SODIUM CATION - UNII:LYR4M0NH37, LACTIC ACID, UNSPECIFIED FORM - UNII:33X04XA5AT) SODIUM LACTATE 450 mg in 100 mL CALCIUM CHLORIDE (UNII: M4I0D6VV5M) (CALCIUM CATION - UNII:2M83C4R6ZB, CHLORIDE ION - UNII:Q32ZN48698) CALCIUM CHLORIDE 25.7 mg in 100 mL MAGNESIUM CHLORIDE (UNII: 02F3473H9O) (MAGNESIUM CATION - UNII:T6V3LHY838, CHLORIDE ION - UNII:Q32ZN48698) MAGNESIUM CHLORIDE 5.1 mg in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0941-0681-04 4 in 1 CARTON 10/21/2024 11/30/2026 1 NDC: 0941-0681-01 2500 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 10/21/2024 11/30/2026 EXTRANEAL
icodextrin, sodium chloride, sodium lactate, calcium chloride, magnesium chloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0941-0717 Route of Administration INTRAPERITONEAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ICODEXTRIN (UNII: 2NX48Z0A9G) (ICODEXTRIN - UNII:2NX48Z0A9G) ICODEXTRIN 7.5 g in 100 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 540 mg in 100 mL SODIUM LACTATE (UNII: TU7HW0W0QT) (SODIUM CATION - UNII:LYR4M0NH37, LACTIC ACID, UNSPECIFIED FORM - UNII:33X04XA5AT) SODIUM LACTATE 450 mg in 100 mL CALCIUM CHLORIDE (UNII: M4I0D6VV5M) (CALCIUM CATION - UNII:2M83C4R6ZB, CHLORIDE ION - UNII:Q32ZN48698) CALCIUM CHLORIDE 25.7 mg in 100 mL MAGNESIUM CHLORIDE (UNII: 02F3473H9O) (MAGNESIUM CATION - UNII:T6V3LHY838, CHLORIDE ION - UNII:Q32ZN48698) MAGNESIUM CHLORIDE 5.1 mg in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0941-0717-05 5 in 1 CARTON 10/21/2024 07/31/2026 1 NDC: 0941-0717-01 2000 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 10/21/2024 07/31/2026 Labeler - Vantive US Healthcare LLC (119181963) Establishment Name Address ID/FEI Business Operations Baxter Healthcare S.A. 988899845 analysis(0941-0681, 0941-0717) , label(0941-0681, 0941-0717) , manufacture(0941-0681, 0941-0717) , pack(0941-0681, 0941-0717) , sterilize(0941-0681, 0941-0717) , api manufacture(0941-0681, 0941-0717)
Trademark Results [EXTRANEAL]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 EXTRANEAL 78566260 not registered Dead/Abandoned |
Baxter International Inc. 2005-02-13 |
 EXTRANEAL 76221877 not registered Dead/Abandoned |
Baxter International Inc. 2001-03-09 |
 EXTRANEAL 76221144 2843444 Live/Registered |
Baxter International Inc. 2001-03-08 |
 EXTRANEAL 75265684 not registered Dead/Abandoned |
Baxter International Inc. 1997-03-28 |
 EXTRANEAL 75250390 not registered Dead/Abandoned |
Baxter International Inc. 1997-03-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.