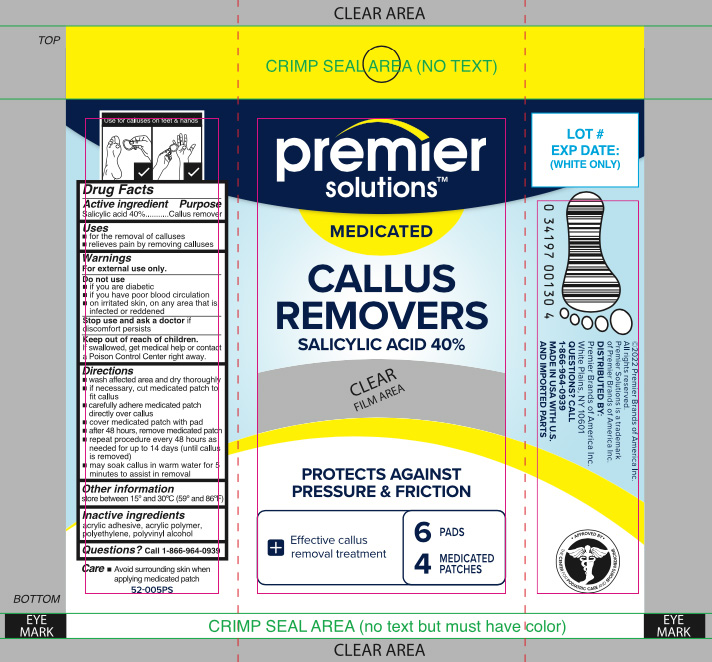
Premier Solutions Callus Removers
Salicylic acid by
Drug Labeling and Warnings
Salicylic acid by is a Otc medication manufactured, distributed, or labeled by Premier Brands of America Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SALICYLIC ACID- callus removers patch
Premier Brands of America Inc.
----------
Premier Solutions Callus Removers
Warnings
For external use only.
Directions
- wash affected area and dry thoroughly
- if necessary, cut medicated patch to fit callus
- carefully adhere medicated patch directly over callus
- cover medicated patch with pad
- after 48 hours, remove medicated patch
- repeat procedure every 48 hours as needed for up to 14 days (until callus is removed)
- may soak callus in warm water for 5 minutes to assist in removal
| SALICYLIC ACID
callus removers patch |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Premier Brands of America Inc. (063849780) |
Revised: 8/2025
Document Id: 3bba69a2-c7c8-e420-e063-6294a90a8741
Set id: e192e81a-b7f9-3461-e053-2995a90a7e2c
Version: 5
Effective Time: 20250806