SENNA SYRUP- sennosides liquid
Senna Syrup by
Drug Labeling and Warnings
Senna Syrup by is a Otc medication manufactured, distributed, or labeled by Safecor Health, LLC, Llorens Pharmaceutical International Division, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Drug Facts
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- ASK DOCTOR
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
1 teaspoonful = 5 mL Directions: - Shake well before use
- Do not exceed recommended dose
Age Starting Dose Maximum Dosage Adults and children 12 years of age and over 2 to 3 teaspoonfuls (10 mL to 15 mL) once a day preferably at bedtime; increase as needed or as recommended by a doctor 3 teaspoonfuls (15 mL) in the morning and 3 teaspoonfuls (15 mL) at bedtime Children under 12 years of age Ask a doctor Ask a doctor - STORAGE AND HANDLING
- INACTIVE INGREDIENT
-
SPL UNCLASSIFIED SECTION
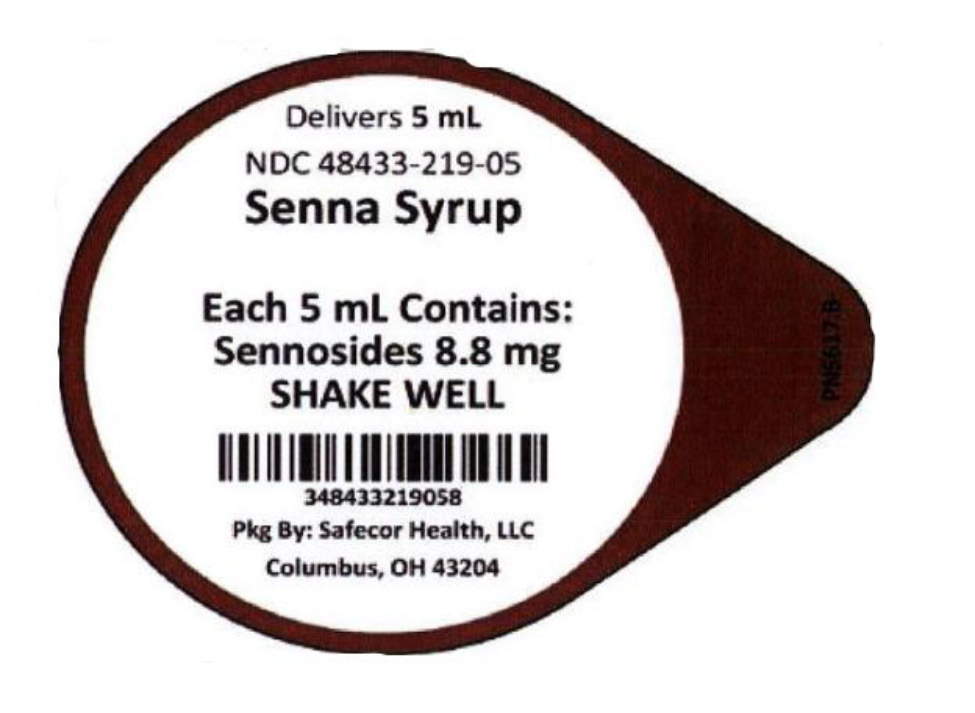
NDC: 48433-219-05 Senna Syrup 8.8 mg/5 mL Unit Dose Cup
Mfd. in the U.S.A.
Distributed by: Safecor Health, LLC
4060 Business Park Drive, Columbus, OH 43204 Rev: 02/2020 PN5497 -
PRINCIPAL DISPLAY PANEL
———Principal Display Panel———
SAFECOR HEALTH
NDC: 48433-219-40
Senna Syrup Qty: 40
8.8 mg Sennosides per 5 mL Lot: 20A0020
Contains 40 (5 mL) Unit Dose Cups Exp: 210203
See monograph for complete drug information.
Store at room temperature 15°C to 25°C (59°F to 77°F);
excursions between 26°C to 30°C (78°F to 86°F) are allowed.
Protect from excessive heat.
This package design is not child resistant. For institutional use only.
Shake well before use.
GTIN: 00348433219409
SN: 200600019
Pkg By: Safecor Health, LLC Columbus, OH 43204 Exp: 210203
Questions or Comments: Call 1-800-447-1006 Lot: 20A0020
-
INGREDIENTS AND APPEARANCE
SENNA SYRUP
sennosides liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 48433-219(NDC:54859-808) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SENNOSIDES (UNII: 3FYP5M0IJX) (SENNOSIDES - UNII:3FYP5M0IJX) SENNOSIDES 8.8 mg in 5 mL Inactive Ingredients Ingredient Name Strength METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SUCRALOSE (UNII: 96K6UQ3ZD4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 48433-219-40 40 in 1 BOX 03/01/2020 1 NDC: 48433-219-05 5 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part334 03/01/2020 Labeler - Safecor Health, LLC (828269675)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.