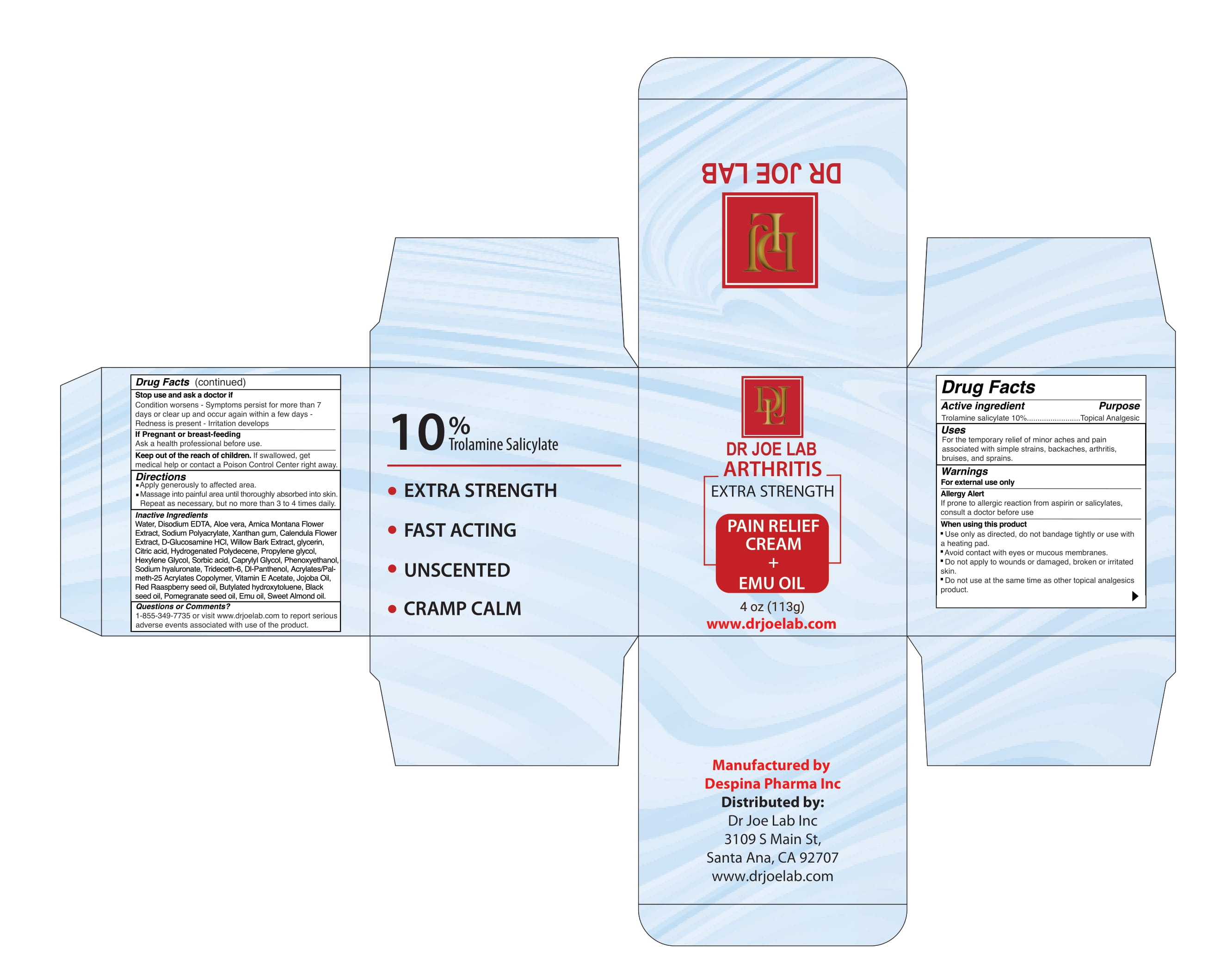
Dr Joe lab ARTHRITIS EXTRA STRENGTH PAIN RELIEF CREAM + EMU OIL 113g
Dr Joe lab ARTHRITIS EXTRA STRENGTH by
Drug Labeling and Warnings
Dr Joe lab ARTHRITIS EXTRA STRENGTH by is a Otc medication manufactured, distributed, or labeled by DESPINA PHARMA INC., Despina Pharma Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
DR JOE LAB ARTHRITIS EXTRA STRENGTH- trolamine salicylate cream
DESPINA PHARMA INC.
----------
Dr Joe lab ARTHRITIS EXTRA STRENGTH PAIN RELIEF CREAM + EMU OIL 113g
uses
For the temporary relief of minor aches and pain
associated with simple strains, backaches, arthritis,
bruises, and sprains.
Warnings
FOR EXTERNAL USE ONLY
Allergy Alert
If prone to allergic reaction from aspirin or salicylates,
consult a doctor before use
When Using
Use only as directed, do not bandage tightly or use with a heating pad.
Avoid contact with eyes or mucous membranes.
Do not apply to wounds or damaged, broken or irritated skin.
Do not use at the same time as other topical analgesics product.
Stop use and ask a doctor if
Condition worsens - Symptoms persist for more than 7
days or clear up and occur again within a few days -
Redness is present - Irritation develops
Keep out of the reach of children. If swallowed, get
medical help or contact a Poison Control Center right away
Apply generously to affected area.
Massage into painful area until thoroughly absorbed into skin.
Repeat as necessary, but no more than 3 to 4 times daily.
Water, Disodium EDTA, Aloe vera, Arnica Montana Flower Extract, Sodium Polyacrylate, Xanthan gum, Calendula Flower Extract, D-Glucosamine HCl, Willow Bark Extract, glycerin, Citric acid, Hydrogenated Polydecene, Propylene glycol, Hexylene Glycol, Sorbic acid, Caprylyl Glycol, Phenoxyethanol, Sodium hyaluronate, Trideceth-6, Dl-Panthenol, Acrylates/Palmeth- 25 Acrylates Copolymer, Vitamin E Acetate, Jojoba Oil, Red Raaspberry seed oil, Butylated hydroxytoluene, Black seed oil, Pomegranate seed oil, Emu oil, Sweet Almond oil.
| DR JOE LAB ARTHRITIS EXTRA STRENGTH
trolamine salicylate cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - DESPINA PHARMA INC. (112281681) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.