MOTION SICKNESS- dimenhydrinate tablet
Motion Sickness by
Drug Labeling and Warnings
Motion Sickness by is a Otc medication manufactured, distributed, or labeled by GREAT LAKES WHOLESALE, MARKETING, & SALES, INC., LNK International, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- difficulty in urination due to an enlargement of the prostate gland
-
Directions
- to prevent motion sickness, the first dose should be taken one-half to one hour before starting activity
adults and children
12 years and over1 to 2 tablets every 4-6 hours;
do not exceed 8 tablets in 24 hours,
or as directed by a doctorchildren 6 to under
12 years
1/2 to 1 tablet every 6-8 hours;
do not exceed 3 tablets in 24 hours,
or as directed by a doctor
children 2 to under
6 years
1/2 tablet every 6-8 hours;
do not exceed 1 1/2 tablets in 24 hours,
or as directed by a doctor
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
HEALTHCARE™
NDC: 64092-600-12
*Compare to the active ingredient
in Dramamine® Original Formula
Motion Sickness
Dimenhydrinate, 50 mg Each
AntiemeticPrevents:
Nausea Dizziness Vomiting
From Motion Sickness12 TABLETS
TAMPER EVIDENT: DO NOT USE IF PACKAGE IS OPENED OR IF BLISTER UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING
*This product is not manufactured or distributed by Medtech Products Inc., owner of the registered trademark Dramamine® Original Formula.
50844 REV0518G19802Distributed by:
Great Lakes Wholesale & Marketing L.L.C.
3729 Patterson Ave., S.E.
Grand Rapids, MI 49512 www.glwholesale.comHEALTHCARE GUARANTEE
If you are not completely satisfied with this
product, regardless of reason, return your unused
portion to Great Lakes Wholesale for a full refund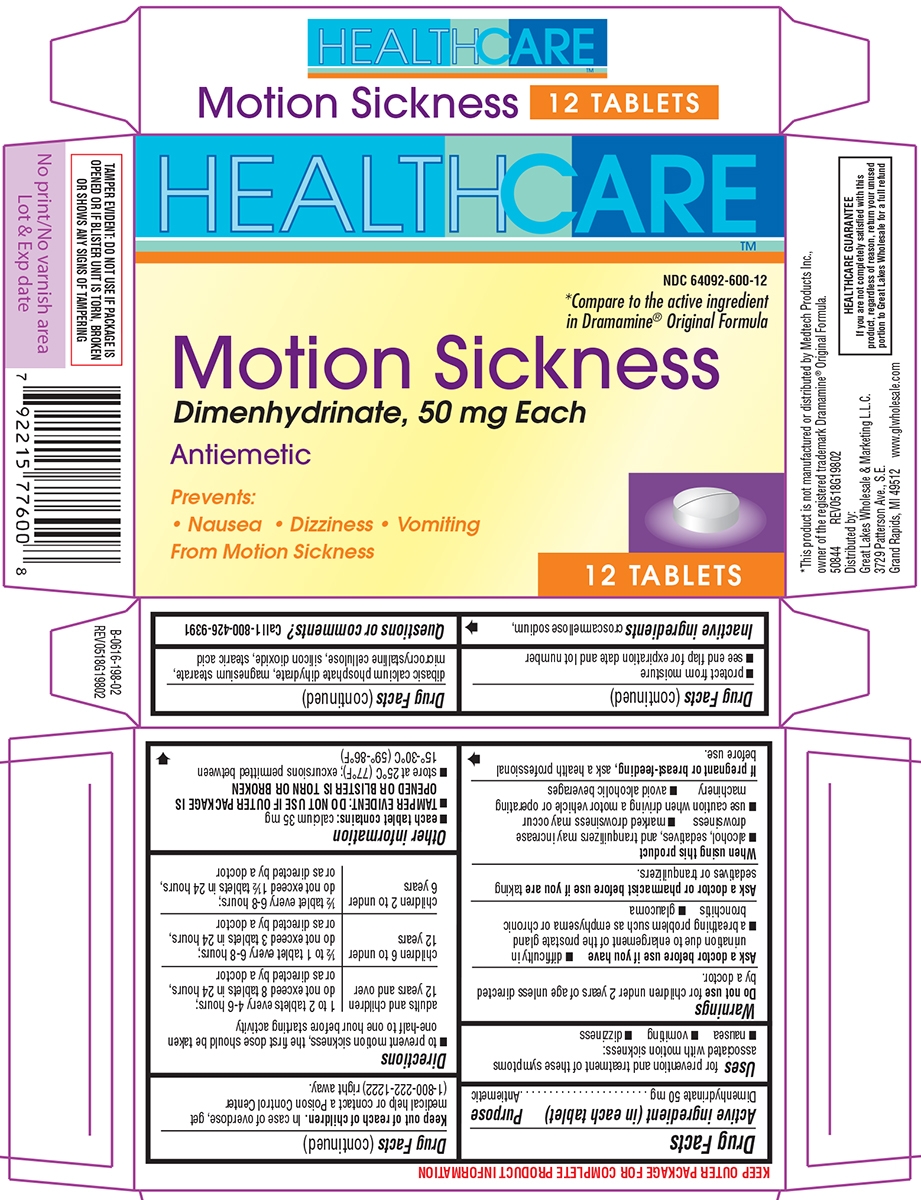
Healthcare 44-198
-
INGREDIENTS AND APPEARANCE
MOTION SICKNESS
dimenhydrinate tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 64092-600 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMENHYDRINATE (UNII: JB937PER5C) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIMENHYDRINATE 50 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) STEARIC ACID (UNII: 4ELV7Z65AP) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) Product Characteristics Color WHITE Score 2 pieces Shape ROUND Size 9mm Flavor Imprint Code 44;198 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 64092-600-12 2 in 1 CARTON 12/01/1992 1 6 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part336 12/01/1992 Labeler - GREAT LAKES WHOLESALE, MARKETING, & SALES, INC. (361925498) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867894 MANUFACTURE(64092-600) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 PACK(64092-600) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 PACK(64092-600) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 PACK(64092-600) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 868734088 PACK(64092-600)
Trademark Results [Motion Sickness]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MOTION SICKNESS 98591174 not registered Live/Pending |
Ronald Joseph Tanksley 2024-06-07 |
 MOTION SICKNESS 87089947 5137431 Live/Registered |
Motion Sickness LLC 2016-06-30 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.