PATADAY- olopatadine hydrochloride solution/ drops
PATADAY by
Drug Labeling and Warnings
PATADAY by is a Prescription medication manufactured, distributed, or labeled by Alcon Laboratories, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use PATADAY™ safely and effectively. See full prescribing information for PATADAY™.
PATADAY™ (olopatadine hydrochloride ophthalmic solution) 0.2%
Initial U.S. Approval: 1996INDICATIONS AND USAGE
PATADAY™ solution is a mast cell stabilizer indicated for the treatment of ocular itching associated with allergic conjunctivitis. (1)
DOSAGE AND ADMINISTRATION
The recommended dose is one drop in each affected eye once a day. (2)
DOSAGE FORMS AND STRENGTHS
Ophthalmic solution 0.2%: each ml contains 2.22 mg of olopatadine hydrochloride. (3)
WARNINGS AND PRECAUTIONS
For topical ocular use only. Not for injection or oral use. (5.1)
ADVERSE REACTIONS
Symptoms similar to cold syndrome and pharyngitis were reported at an incidence of approximately 10%. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Alcon Laboratories, Inc. at 1-800-757-9195 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 12/2010
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 For topical ocular use only.
5.2 Contamination of Tip and Solution
5.3 Contact Lens Use
6 ADVERSE REACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
17.1 Topical Ophthalmic Use Only
17.2 Sterility of Dropper Tip
17.3 Concomitant Use of Contact Lenses
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
- 2 DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.2 Contamination of Tip and Solution
As with any eye drop, to prevent contaminating the dropper tip and solution, care should be taken not to touch the eyelids or surrounding areas with the dropper tip of the bottle. Keep bottle tightly closed when not in use.
5.3 Contact Lens Use
Patients should be advised not to wear a contact lens if their eye is red.
PATADAY™ (olopatadine hydrochloride ophthalmic solution) 0.2% should not be used to treat contact lens related irritation.
The preservative in PATADAY™ solution, benzalkonium chloride, may be absorbed by soft contact lenses. Patients who wear soft contact lenses and whose eyes are not red, should be instructed to wait at least ten minutes after instilling PATADAY™ (olopatadine hydrochloride ophthalmic solution) 0.2% before they insert their contact lenses.
-
6 ADVERSE REACTIONS
Symptoms similar to cold syndrome and pharyngitis were reported at an incidence of approximately 10%.
The following adverse experiences have been reported in 5% or less of patients:
Ocular: blurred vision, burning or stinging, conjunctivitis, dry eye, foreign body sensation, hyperemia, hypersensitivity, keratitis, lid edema, pain and ocular pruritus.
Non-ocular: asthenia, back pain, flu syndrome, headache, increased cough, infection, nausea, rhinitis, sinusitis and taste perversion.
Some of these events were similar to the underlying disease being studied.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic effects: Pregnancy Category C
Olopatadine was found not to be teratogenic in rats and rabbits. However, rats treated at 600 mg/kg/day, or 150,000 times the MROHD and rabbits treated at 400 mg/kg/day, or approximately 100,000 times the MROHD, during organogenesis showed a decrease in live fetuses. In addition, rats treated with 600 mg/kg/day of olopatadine during organogenesis showed a decrease in fetal weight. Further, rats treated with 600 mg/kg/day of olopatadine during late gestation through the lactation period showed a decrease in neonatal survival and body weight. There are, however, no adequate and well- controlled studies in pregnant women. Because animal studies are not always predictive of human responses, this drug should be used in pregnant women only if the potential benefit to the mother justifies the potential risk to the embryo or fetus.
8.3 Nursing Mothers
Olopatadine has been identified in the milk of nursing rats following oral administration. It is not known whether topical ocular administration could result in sufficient systemic absorption to produce detectable quantities in the human breast milk. Nevertheless, caution should be exercised when PATADAY™ (olopatadine hydrochloride ophthalmic solution) 0.2% is administered to a nursing mother.
-
11 DESCRIPTION
PATADAY™ (olopatadine hydrochloride ophthalmic solution) 0.2% is a sterile ophthalmic solution containing olopatadine for topical administration to the eyes. Olopatadine hydrochloride is a white, crystalline, water-soluble powder with a molecular weight of 373.88 and a molecular formula of C21H23NO3 HCl.
The chemical structure is presented below:
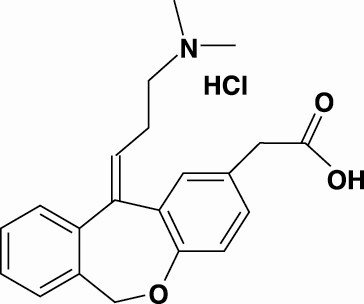
Chemical Name: 11-[(Z)-3-(Dimethylamino) propylidene]-6-11-dihydrodibenz[b,e] oxepin-2-acetic acid, hydrochloride
Each mL of PATADAY™ solution contains: Active: 2.22 mg olopatadine hydrochloride equivalent to 2 mg olopatadine. Inactives: povidone; dibasic sodium phosphate; sodium chloride; edetate disodium; benzalkonium chloride 0.01% (preservative); hydrochloric acid/sodium hydroxide (adjust pH); and purified water.
It has a pH of approximately 7 and an osmolality of approximately 300 mOsm/kg.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Olopatadine is a mast cell stabilizer and a histamine H1 antagonist. Decreased chemotaxis and inhibition of eosinophil activation has also been demonstrated.
12.3 Pharmacokinetics
Systemic bioavailability data upon topical ocular administration of PATADAY™ solution are not available. Following topical ocular administration of olopatadine 0.15% ophthalmic solution in man, olopatadine was shown to have a low systemic exposure. Two studies in normal volunteers (totaling 24 subjects) dosed bilaterally with olopatadine 0.15% ophthalmic solution once every 12 hours for 2 weeks demonstrated plasma concentrations to be generally below the quantitation limit of the assay (< 0.5 ng/mL). Samples in which olopatadine was quantifiable were typically found within 2 hours of dosing and ranged from 0.5 to 1.3 ng/mL. The elimination half-life in plasma following oral dosing was 8 to 12 hours, and elimination was predominantly through renal excretion. Approximately 60 - 70% of the dose was recovered in the urine as parent drug. Two metabolites, the mono-desmethyl and the N-oxide, were detected at low concentrations in the urine.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Olopatadine administered orally was not carcinogenic in mice and rats in doses up to 500 mg/kg/day and 200 mg/kg/day, respectively. Based on a 40 μL drop size and a 50 kg person, these doses were approximately 150,000 and 50,000 times higher than the maximum recommended ocular human dose (MROHD). No mutagenic potential was observed when olopatadine was tested in an in vitro bacterial reverse mutation (Ames) test, an in vitro mammalian chromosome aberration assay or an in vivo mouse micronucleus test. Olopatadine administered to male and female rats at oral doses of approximately 100,000 times MROHD level resulted in a slight decrease in the fertility index and reduced implantation rate; no effects on reproductive function were observed at doses of approximately 15,000 times the MROHD level.
- 14 CLINICAL STUDIES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
PATADAY™ (olopatadine hydrochloride ophthalmic solution) 0.2% is supplied in a white, oval, low density polyethylene DROP-TAINER® dispenser with a natural low density polyethylene dispensing plug and a white polypropylene cap. Tamper evidence is provided with a shrink band around the closure and neck area of the package.
2.5 mL fill in 4 mL bottle (NDC: 0065-0272-25)
Storage
Store at 2°C to 25°C (36°F to 77°F)
-
17 PATIENT COUNSELING INFORMATION
17.2 Sterility of Dropper Tip
Patients should be advised to not touch dropper tip to any surface, as this may contaminate the contents.
17.3 Concomitant Use of Contact Lenses
Patients should be advised not to wear a contact lens if their eyes are red. Patients should be advised that PATADAY™ solution should not be used to treat contact lens-related irritation. Patients should also be advised to remove contact lenses prior to instillation of PATADAY™ solution. The preservative in PATADAY™ solution benzalkonium chloride may be absorbed by soft contact lenses. Lenses may be reinserted following administration of PATADAY™ solution.
U.S. Patents Nos. 5,641,805; 6,995,186; 7,402,609
9010558-0314
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PATADAY
olopatadine hydrochloride solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0065-0272 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OLOPATADINE HYDROCHLORIDE (UNII: 2XG66W44KF) (OLOPATADINE - UNII:D27V6190PM) OLOPATADINE 2 mg in 1 mL Inactive Ingredients Ingredient Name Strength POVIDONE (UNII: FZ989GH94E) SODIUM PHOSPHATE, DIBASIC (UNII: GR686LBA74) SODIUM CHLORIDE (UNII: 451W47IQ8X) EDETATE DISODIUM (UNII: 7FLD91C86K) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0065-0272-25 1 in 1 CARTON 02/15/2007 1 2.5 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 2 NDC: 0065-0272-11 1 in 1 CARTON 02/15/2007 2 1 in 1 POUCH 2 0.5 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021545 02/15/2007 Labeler - Alcon Laboratories, Inc. (008018525)
Trademark Results [PATADAY]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PATADAY 78844293 4332241 Live/Registered |
NOVARTIS AG 2006-03-23 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
