BENZTROPINE MESYLATE tablet
BENZTROPINE MESYLATE by
Drug Labeling and Warnings
BENZTROPINE MESYLATE by is a Prescription medication manufactured, distributed, or labeled by QUAGEN PHARMACEUTICALS LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
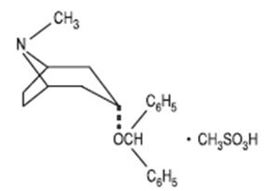
Benztropine mesylate, USP is a synthetic compound containing structural features found in atropine and diphenhydramine.
It is a crystalline white powder, very soluble in water, designated as 3α- (Diphenylmethoxy)-1αH, 5αH-tropane methanesulfonate, with the following structural formula:
Each benztropine mesylate tablet, USP for oral administration contains benztropine mesylate 0.5 mg, 1 mg or 2 mg.
Each tablet contains the following inactive ingredients: microcrystalline cellulose, pregelatinized starch, magnesium stearate.
-
CLINICAL PHARMACOLOGY
Benztropine mesylate possesses both anticholinergic and antihistaminic effects, although only the former have been established as therapeutically significant in the management of parkinsonism.
In the isolated guinea pig ileum, the anticholinergic activity of this drug is about equal to that of atropine; however, when administered orally to unanesthetized cats, it is only about half as active as atropine.
In laboratory animals, its antihistaminic activity and duration of action approach those of pyrilamine maleate.
-
INDICATIONS AND USAGE
Benztropine mesylate tablets are indicated for use as an adjunct in the therapy of all forms of parkinsonism.
Useful also in the control of extrapyramidal disorders [except tardive dyskinesia - see PRECAUTIONS] due to neuroleptic drugs (e.g., phenothiazines).
- CONTRAINDICATIONS
-
WARNINGS
Safe use in pregnancy has not been established.
Benztropine mesylate may impair mental and/or physical abilities required for performance of hazardous tasks, such as operating machinery or driving a motor vehicle.
When benztropine mesylate is given concomitantly with phenothiazines, haloperidol, or other drugs with anticholinergic or antidopaminergic activity, patients should be advised to report gastrointestinal complaints, fever or heat intolerance promptly. Paralytic ileus, hyperthermia and heat stroke, all of which have sometimes been fatal, have occurred in patients taking anticholinergic-type antiparkinsonism drugs, including benztropine mesylate, in combination with phenothiazines and/or tricyclic antidepressants.
Since benztropine mesylate contains structural features of atropine, it may produce anhidrosis. For this reason, it should be administered with caution during hot weather, especially when given concomitantly with other atropine-like drugs to the chronically ill, the alcoholic, those who have central nervous system disease, and those who do manual labor in a hot environment. Anhidrosis may occur more readily when some disturbance of sweating already exists. If there is evidence of anhidrosis, the possibility of hyperthermia should be considered. Dosage should be decreased at the discretion of the physician so that the ability to maintain body heat equilibrium by perspiration is not impaired. Severe anhidrosis and fatal hyperthermia have occurred.
-
PRECAUTIONS
General
Since benztropine mesylate has cumulative action, continued supervision is advisable. Patients with a tendency to tachycardia and patients with prostatic hypertrophy should be observed closely during treatment.
Dysuria may occur, but rarely becomes a problem. Urinary retention has been reported with benztropine mesylate.
The drug may cause complaints of weakness and inability to move particular muscle groups, especially in large doses. For example, if the neck has been rigid and suddenly relaxes, it may feel weak, causing some concern. In this event, dosage adjustment is required.
Mental confusion and excitement may occur with large doses, or in susceptible patients. Visual hallucinations have been reported occasionally. Furthermore, in the treatment of extrapyramidal disorders due to neuroleptic drugs (e.g., phenothiazines), in patients with mental disorders, occasionally there may be intensification of mental symptoms. In such cases, antiparkinsonian drugs can precipitate a toxic psychosis. Patients with mental disorders should be kept under careful observation, especially at the beginning of treatment or if dosage is increased.
Tardive dyskinesia may appear in some patients on long-term therapy with phenothiazines and related agents, or may occur after therapy with these drugs has been discontinued. Antiparkinsonism agents do not alleviate the symptoms of tardive dyskinesia, and in some instances may aggravate them. Benztropine mesylate is not recommended for use in patients with tardive dyskinesia.
The physician should be aware of the possible occurrence of glaucoma. Although the drug does not appear to have any adverse effect on simple glaucoma, it probably should not be used in angle-closure glaucoma.
Drug Interactions
Antipsychotic drugs such as phenothiazines or haloperidol; tricyclic antidepressants [see WARNINGS].
Pediatric Use
Because of the atropine-like side effects, benztropine mesylate should be used with caution in pediatric patients over three years of age [see CONTRAINDICATIONS].
-
ADVERSE REACTIONS
The adverse reactions below, most of which are anticholinergic in nature, have been reported and within each category are listed in order of decreasing severity.
Cardiovascular
Tachycardia.Digestive
Paralytic ileus, constipation, vomiting, nausea, dry mouth.If dry mouth is so severe that there is difficulty in swallowing or speaking, or loss of appetite and weight, reduce dosage, or discontinue the drug temporarily.
Slight reduction in dosage may control nausea and still give sufficient relief of symptoms. Vomiting may be controlled by temporary discontinuation, followed by resumption at a lower dosage.
Nervous System
Toxic psychosis, including confusion, disorientation, memory impairment, visual hallucinations; exacerbation of preexisting psychotic symptoms; nervousness; depression; listlessness; numbness of fingers.Special Senses
Blurred vision, dilated pupils.Urogenital
Urinary retention, dysuria.Metabolic/Immune or Skin
Occasionally, an allergic reaction, e.g., skin rash, develops. If this can not be controlled by dosage reduction, the medication should be discontinued.Other
Heat stroke, hyperthermia, fever. -
OVERDOSAGE
Manifestations - May be any of those seen in atropine poisoning or antihistamine overdosage: CNS depression, preceded or followed by stimulation; confusion; nervousness; listlessness; intensification of mental symptoms or toxic psychosis in patients with mental illness being treated with neuroleptic drugs (e.g., phenothiazines); hallucinations (especially visual); dizziness; muscle weakness; ataxia; dry mouth; mydriasis; blurred vision; palpitations; tachycardia; elevated blood pressure; nausea; vomiting; dysuria; numbness of fingers; dysphagia; allergic reactions, e.g., skin rash; headache; hot, dry, flushed skin; delirium; coma; shock; convulsions; respiratory arrest; anhidrosis; hyperthermia; glaucoma; constipation.
Treatment - Physostigmine salicylate, 1 mg to 2 mg, SC or IV, reportedly will reverse symptoms of anticholinergic intoxication.1 A second injection may be given after 2 hours if required. Otherwise treatment is symptomatic and supportive. Induce emesis or perform gastric lavage (contraindicated in precomatose, convulsive, or psychotic states). Maintain respiration. A short-acting barbiturate may be used for CNS excitement, but with caution to avoid subsequent depression; supportive care for depression (avoid convulsant stimulants such as picrotoxin, pentylenetetrazol, or bemegride); artificial respiration for severe respiratory depression; a local miotic for mydriasis and cycloplegia; ice bags or other cold applications and alcohol sponges for hyperpyrexia, a vasopressor and fluids for circulatory collapse. Darken room for photophobia.
- 1 Duvoisin, R.C.; Katz, R.J.; Amer. Med. Ass. 206: 1963-1965, Nov. 25, 1968.
-
DOSAGE AND ADMINISTRATION
Benztropine mesylate tablets should be used when patients are able to take oral medication.
The injection is especially useful for psychotic patients with acute dystonic reactions or other reactions that make oral medication difficult or impossible. It is recommended also when a more rapid response is desired than can be obtained with the tablets.
Because of cumulative action, therapy should be initiated with a low dose which is increased gradually at five or six-day intervals to the smallest amount necessary for optimal relief. Increases should be made in increments of 0.5 mg, to a maximum of 6 mg, or until optimal results are obtained without excessive adverse reactions.
Postencephalitic and Idiopathic Parkinsonism - The usual daily dose is 1 mg to 2 mg, with a range of 0.5 mg to 6 mg orally or parenterally.
As with any agent used in parkinsonism, dosage must be individualized according to age and weight, and the type of parkinsonism being treated. Generally, older patients, and thin patients cannot tolerate large doses. Most patients with postencephalitic parkinsonism need fairly large doses and tolerate them well. Patients with a poor mental outlook are usually poor candidates for therapy.
In idiopathic parkinsonism, therapy may be initiated with a single daily dose of 0.5 mg to 1 mg at bedtime. In some patients, this will be adequate; in others 4 mg to 6 mg a day may be required.
In postencephalitic parkinsonism, therapy may be initiated in most patients with 2 mg a day in one or more doses. In highly sensitive patients, therapy may be initiated with 0.5 mg at bedtime, and increased as necessary.
Some patients experience greatest relief by taking the entire dose at bedtime; others react more favorably to divided doses, two to four times a day. Frequently, one dose a day is sufficient, and divided doses may be unnecessary or undesirable.
The long duration of action of this drug makes it particularly suitable for bedtime medication when its effects may last throughout the night, enabling patients to turn in bed during the night more easily, and to rise in the morning.
When benztropine mesylate is started, do not terminate therapy with other antiparkinsonian agents abruptly. If the other agents are to be reduced or discontinued, it must be done gradually. Many patients obtain greatest relief with combination therapy.
Benztropine mesylate tablets may be used concomitantly with Carbidopa-Levodopa, or with levodopa, in which case periodic dosage adjustment may be required in order to maintain optimum response.
Drug-Induced Extrapyramidal Disorders - In treating extrapyramidal disorders due to neuroleptic drugs (e.g., phenothiazines), the recommended dosage is 1 mg to 4 mg once or twice a day orally. Dosage must be individualized according to the need of the patient. Some patients require more than recommended; others do not need as much.
When extrapyramidal disorders develop soon after initiation of treatment with neuroleptic drugs (e.g., phenothiazines), they are likely to be transient. One to 2 mg of benztropine mesylate tablets two or three times a day usually provides relief within one or two days. After one or two weeks, the drug should be withdrawn to determine the continued need for it. If such disorders recur, benztropine mesylate can be reinstituted.
Certain drug-induced extrapyramidal disorders that develop slowly may not respond to benztropine mesylate.
-
HOW SUPPLIED
Benztropine Mesylate Tablets, USP, for oral use, are supplied in the following forms:
0.5 mg: White to off-white, round, flat faced bevel edged tablets debossed "T" above "14" below the bisect on one side and plain on the other side. They are supplied as follows.
Bottles of 100 tablets
NDC: 70752-123-10
Bottles of 1000 tablets
NDC: 70752-123-11
1 mg: White to off-white, oval shape, tablets debossed "T" to the left and "15" to the right of the bisect on one side and plain on the other side. They are supplied as follows.
Bottles of 100 tablets
NDC: 70752-124-10
Bottles of 1000 tablets
NDC: 70752-124-11
2 mg: White to off-white, round, flat faced bevel edged tablets debossed "T" above "16" below the bisect on one side and plain on the other side. They are supplied as follows.
Bottles of 100 tablets
NDC: 70752-125-10
Bottles of 1000 tablets
NDC: 70752-125-11
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature].
Dispense in well-closed containers as defined in the USP.
Keep out of reach of children.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 0.5 mg Tablet Bottle Label
- PRINCIPAL DISPLAY PANEL - 1 mg Tablet Bottle Label
- PRINCIPAL DISPLAY PANEL - 2 mg Tablet Bottle Label
-
INGREDIENTS AND APPEARANCE
BENZTROPINE MESYLATE
benztropine mesylate tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70752-123 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZTROPINE MESYLATE (UNII: WMJ8TL7510) (BENZTROPINE - UNII:1NHL2J4X8K) BENZTROPINE MESYLATE 0.5 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, PREGELATINIZED CORN (UNII: O8232NY3SJ) Product Characteristics Color WHITE (White to Off-White) Score 2 pieces Shape ROUND (flat beveled edge) Size 6mm Flavor Imprint Code T;14 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70752-123-10 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/11/2025 2 NDC: 70752-123-11 1000 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/11/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA212694 08/11/2025 BENZTROPINE MESYLATE
benztropine mesylate tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70752-124 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZTROPINE MESYLATE (UNII: WMJ8TL7510) (BENZTROPINE - UNII:1NHL2J4X8K) BENZTROPINE MESYLATE 1 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, PREGELATINIZED CORN (UNII: O8232NY3SJ) Product Characteristics Color WHITE (White to Off-White) Score 2 pieces Shape OVAL Size 11mm Flavor Imprint Code T;15 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70752-124-10 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/11/2025 2 NDC: 70752-124-11 1000 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/11/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA212694 08/11/2025 BENZTROPINE MESYLATE
benztropine mesylate tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70752-125 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZTROPINE MESYLATE (UNII: WMJ8TL7510) (BENZTROPINE - UNII:1NHL2J4X8K) BENZTROPINE MESYLATE 2 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, PREGELATINIZED CORN (UNII: O8232NY3SJ) Product Characteristics Color WHITE (White to Off-White) Score 2 pieces Shape ROUND (flat beveled edge) Size 7mm Flavor Imprint Code T;16 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70752-125-10 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/11/2025 2 NDC: 70752-125-11 1000 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/11/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA212694 08/11/2025 Labeler - QUAGEN PHARMACEUTICALS LLC (073645339) Registrant - QUAGEN PHARMACEUTICALS LLC (073645339) Establishment Name Address ID/FEI Business Operations QUAGEN PHARMACEUTICALS LLC 081343942 manufacture(70752-123, 70752-124, 70752-125) , pack(70752-123, 70752-124, 70752-125)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.


