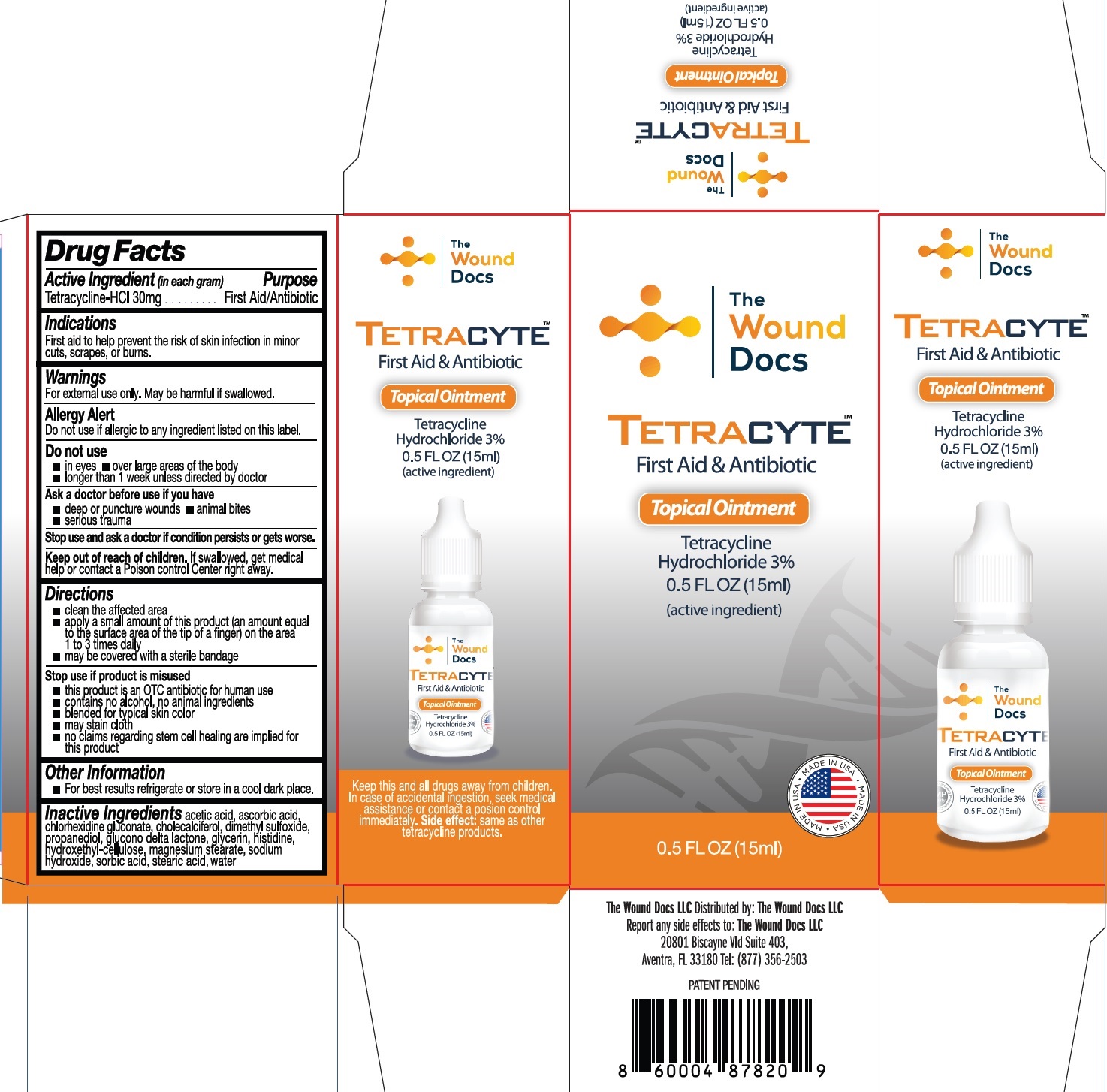
TETRACYTE TOPICAL - tetracycline hydrochloride ointment
TETRACYTE TOPICAL tetracycline hydrochloride by
Drug Labeling and Warnings
TETRACYTE TOPICAL tetracycline hydrochloride by is a Otc medication manufactured, distributed, or labeled by THE WOUND DOCS, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
TETRACYTE TOPICAL TETRACYCLINE HYDROCHLORIDE- tetracycline hydrochloride ointment
THE WOUND DOCS, LLC
----------
TETRACYTE TOPICAL - tetracycline hydrochloride ointment
Warnings
For external use only. May be harmful if swallowed.
Allergy Alert
Do not use if allergic to any ingredient listed on this label.
Directions
clean the affected area
apply a small amount of this product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily
may be covered with a sterile bandage
Stop use if product is misused
this product is an OTC antibiotic for human use
contains no alcohol, no animal ingredients
blended for typical skin color
may stain cloth
no claims regarding stem cell healing are implied for this product
| TETRACYTE TOPICAL TETRACYCLINE HYDROCHLORIDE
tetracycline hydrochloride ointment |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - THE WOUND DOCS, LLC (118705435) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.