OVUGEL- triptorelin acetate gel
OvuGel by
Drug Labeling and Warnings
OvuGel by is a Animal medication manufactured, distributed, or labeled by Aurora Pharmaceutical, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT:
- DOSAGE FORM & STRENGTH:
- INDICATIONS FOR USE:
- DESCRIPTION:
-
WARNINGS:
WITHDRAWAL PERIOD:
No withdrawal period is required when used according to labeling.
USER SAFETY WARNINGS:Not for Use in Humans. Keep Out of Reach of Children. The Material Safety Data Sheet (MSDS) contains more detailed occupational safety information.
ANIMAL SAFETY WARNINGS:OvuGel® should not be used in sows with obvious reproductive tract abnormalities.
- RECOMMENDATIONS FOR SAFE AND EFFECTIVE USE:
-
DIRECTIONS FOR USE:
OvuGel® should be administered intravaginally at approximately 96 hours after weaning. The product should be warmed at room temperature for a minimum of 10 minutes prior to use. Insert the delivery tube into the vagina so that the tip rests 1/2 inch (1-3 cm) posterior to the cervix. Use a separate sheath over the delivery tube for each sow treated. Each sow should receive a single 2 mL dose of OvuGel®. Sows should be inseminated 22 +/- 2 hours following administration of OvuGel® using standard artificial insemination techniques. Sows should be exposed to a boar during time of insemination. (See end of package insert for more detailed directions for OvuGel® administration).
-
STORAGE, HANDLING, AND DISPOSAL:
Store at refrigerator temperature 36-46°F (2-8°C). Excursions permitted to 77°F (25°C) for no more than 5 days during shipping.
OvuGel® should be administered within 1 hour of warming; unused portions may be refrigerated immediately after use. The bottle stopper may be punctured a maximum of three times. Discard 28 days after the stopper is first punctured. - HOW SUPPLIED:
-
QUESTIONS/COMMENTS
To obtain an MSDS, for technical assistance, or to report adverse events, contact Aurora Pharmaceutical at 1-888-215-1256 or www.aurorapharmaceutical.com. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS, or www.fda.gov/reportanimalae.
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel – 52.5 mL Bottle Carton
OvuGel®
(triptorelin acetate)
100 mcg triptorelin per mL (as triptorelin acetate)
Gel for intravaginal useINDICATIONS FOR USE:
For the synchronization of time of insemination in weaned sows to facilitate a single fixed-time artificial insemination.Not approved for use in gilts. Safety and effectiveness have not been evaluated in these animals.
Net Contents: 52.5 mL
multi-dose bottle (26 doses, 2 mL each)Approved by FDA under NADA # 141-339
NDC: 51072-087-00Manufactured by: Aurora Pharmaceutical, Inc.
1196 Hwy 3 S
Northfield, MN 55057IN 40-1859 Rev. 02/2025
Reorder No: 59030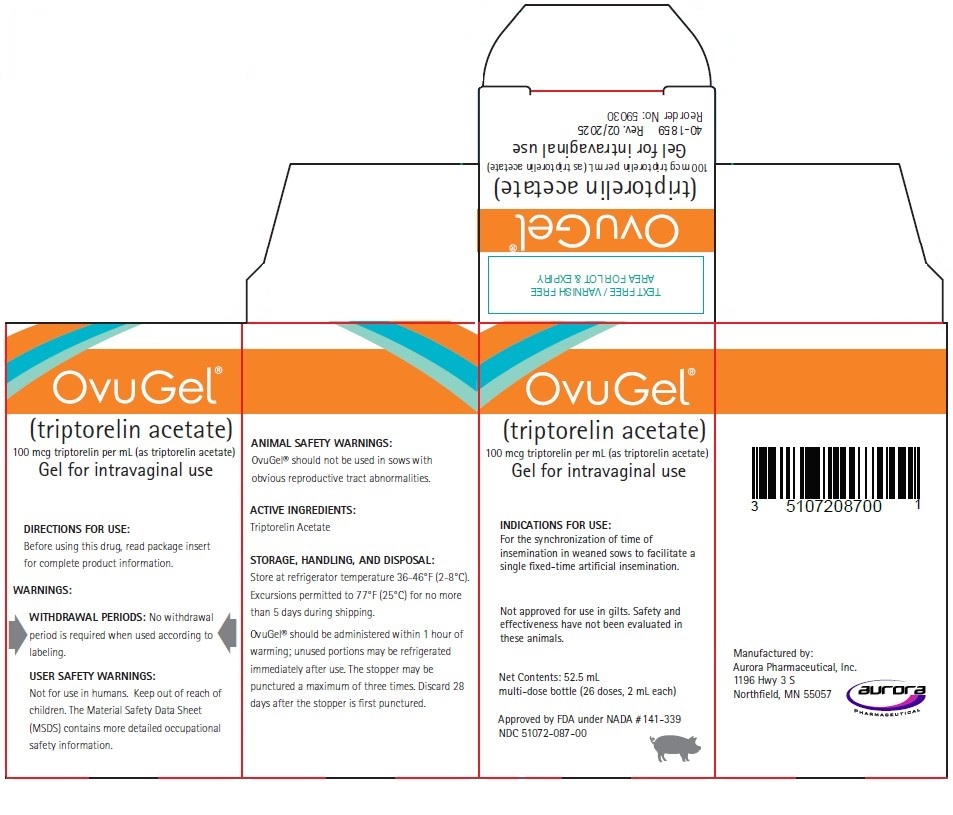
-
INGREDIENTS AND APPEARANCE
OVUGEL
triptorelin acetate gelProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC: 51072-087 Route of Administration VAGINAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Triptorelin Acetate (UNII: 43OFW291R9) (Triptorelin - UNII:9081Y98W2V) Triptorelin 100 ug in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51072-087-00 1 in 1 CARTON 1 52.5 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141339 01/20/2026 Labeler - Aurora Pharmaceutical, Inc. (832848639) Establishment Name Address ID/FEI Business Operations Aurora Pharmaceutical, Inc. 832848639 manufacture
Trademark Results [OvuGel]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 OVUGEL 78175599 not registered Dead/Abandoned |
EiEiCo, Inc. 2002-10-17 |
 OVUGEL 77674437 not registered Dead/Abandoned |
L. Perrigo Company 2009-02-20 |
 OVUGEL 77275688 3835525 Live/Registered |
UNITED ANIMAL HEALTH, INC. 2007-09-10 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.