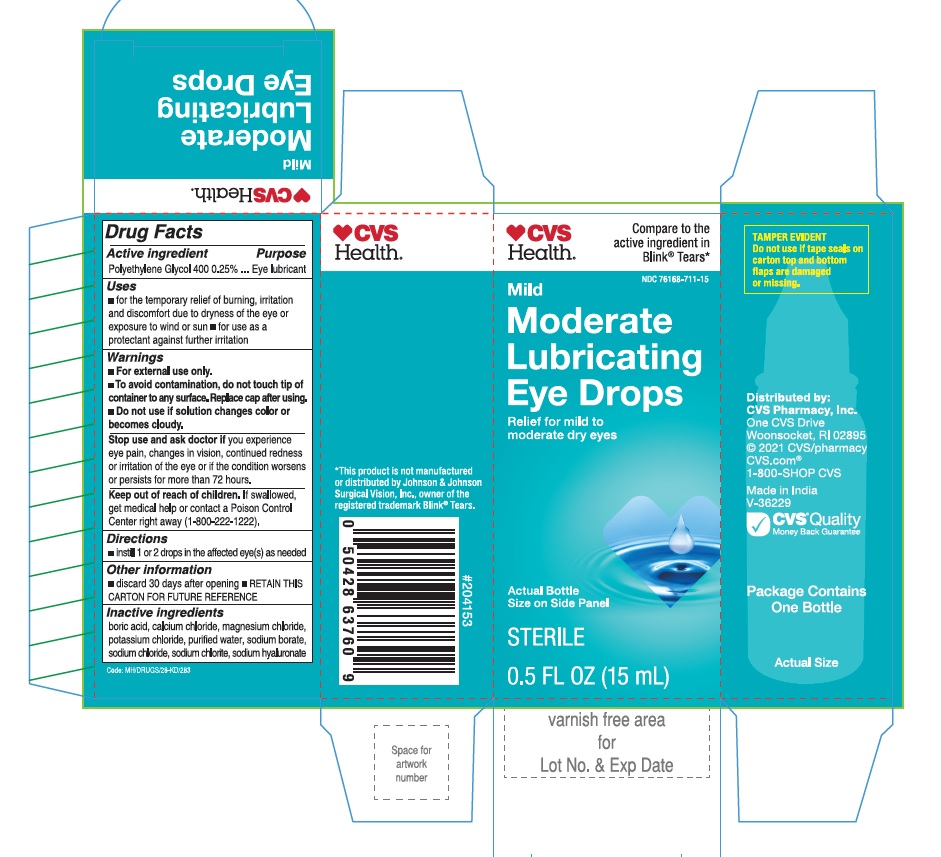
Moderate lubricating drops by Velocity Pharma LLC
Moderate lubricating drops by
Drug Labeling and Warnings
Moderate lubricating drops by is a Otc medication manufactured, distributed, or labeled by Velocity Pharma LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
MODERATE LUBRICATING DROPS CVS HEALTH- polyethylene glycol 400 solution/ drops
Velocity Pharma LLC
----------
Uses
- For the temporary relief of burning, irritation and discomfort due to dryness of the eye or exposure to wind or sun.
- May be used as a protectant against further irritation.
WARNINGS
- For external use only.
- To avoid contamination, do not touch tip of container to any surface. Replace cap after using.
- Do not use if solution changes color or becomes cloudy.
Stop use and ask doctor if:
You experience eye pain, changes in vision, continued redness or irritation of the eye, or if the condition worsens or persists for more than 72 hours.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
If swallowed, get medical help of contact a Poison Control Center right away.
| MODERATE LUBRICATING DROPS
CVS HEALTH
polyethylene glycol 400 solution/ drops |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Velocity Pharma LLC (962198409) |
Revised: 1/2024
Document Id: 0fa853a8-61a8-5e6d-e063-6294a90a38ee
Set id: e2016198-48ce-9cbc-e053-2995a90ac47d
Version: 2
Effective Time: 20240123
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.