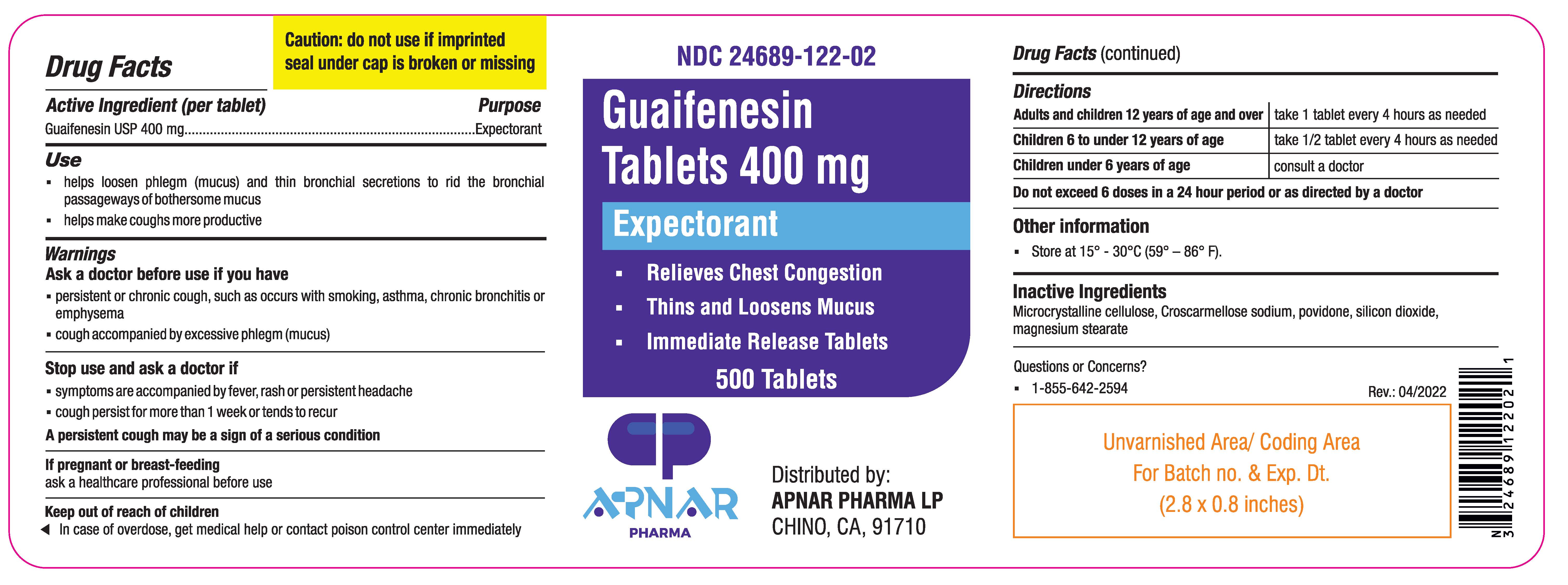
Guaifenesin Tablets 400 mg
Guaifenesin by
Drug Labeling and Warnings
Guaifenesin by is a Otc medication manufactured, distributed, or labeled by APNAR PHARMA LP, Apnar Pharma Private Limited, APNAR PHARMA LLP. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
GUAIFENESIN- guaifenesin tablet
APNAR PHARMA LP
----------
Guaifenesin Tablets 400 mg
Use
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus
- helps make coughs more productive
Warnings
Ask doctor before use if you have
- persistent or chronic cough, such as occurs with smoking, asthma, bronchitis or emphysema
- cough accompanied by excessive phlegm (mucus)
Stop use and ask a doctor if
- Symptoms are accompanied by fever, rash or persistent headache
- cough persists for more than 1 week or tends to recur
- A persistent cough may be a sign of a serious condition
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center immediately.
Directions
- Adults and children 12 years of age and over: take 1 tablet every 4 hours as needed
- Children 6 to 12 years of age: take 1/2 tablet every 4 hours as needed
- Children under 6 years of age: consult a doctor
Do not exceed 6 doses in a 24 hour period or as directed by a doctor
| GUAIFENESIN
guaifenesin tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - APNAR PHARMA LP (079568229) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| APNAR PHARMA LLP | 118530917 | pack(24689-122) , label(24689-122) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Apnar Pharma Private Limited | 876730408 | analysis(24689-122) , manufacture(24689-122) | |
Revised: 12/2025
Document Id: 45d2f472-0372-f59c-e063-6394a90ab537
Set id: e217672e-ba0a-e79e-e053-2a95a90a6cd1
Version: 5
Effective Time: 20251213
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.