VIVJOA- oteseconazole capsule
VIVJOA by
Drug Labeling and Warnings
VIVJOA by is a Prescription medication manufactured, distributed, or labeled by Mycovia Pharmaceuticals, Inc., Patheon, Inc. (a ThermoFisher Company), Patheon, Inc., Packaging Coordinators, LLC., Evonik Corporation Tippecanoe Laboratories, Micro-macinazione SA Lonza Pharm and Biotech, Dynamit Nobel GmBH Explosivoff-und Systemtechnik (Novasep), ChemCon, Currenta GmBH &Co OHG, BioChem Labor fur biologische und chemische Analytik GmBH. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use VIVJOA™ safely and effectively. See full prescribing information for VIVJOA™.
VIVJOA™ (oteseconazole) capsules, for oral use
Initial U.S. Approval: 2022INDICATIONS AND USAGE
VIVJOA™ is an azole antifungal indicated to reduce the incidence of recurrent vulvovaginal candidiasis (RVVC) in females with a history of RVVC who are NOT of reproductive potential. ( 1)
DOSAGE AND ADMINISTRATION
- There are two recommended VIVJOA dosage regimens: a VIVJOA-only regimen and a Fluconazole/VIVJOA regimen. Use one of these two dosage regimens. (
2.1)
- Administer VIVJOA orally with food. ( 2.1)
- For the
VIVJOA-only Dosage Regimen: (
2.2)
- On Day 1:Administer VIVJOA 600 mg (as a single dose), then
- On Day 2:Administer VIVJOA 450 mg (as a single dose), then
- Beginning on Day 14: Administer VIVJOA 150 mg once a week (every 7 days) for 11 weeks (Weeks 2 through 12).
- For the
Fluconazole/VIVJOA Dosage Regimen, prescribe fluconazole and: (
2.3)
- On Day 1, Day 4, and Day 7: Administer fluconazole 150 mg orally,then
- On Days 14 through 20: Administer VIVJOA 150 mg once daily for 7 days, then
- Beginning on Day 28: Administer VIVJOA 150 mg once a week (every 7 days) for 11 weeks (Weeks 4 through 14).
DOSAGE FORMS AND STRENGTHS
Capsules:150 mg of oteseconazole (fluconazole is not supplied in the carton). ( 3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
Embryo-Fetal Toxicity:Based on animal studies, VIVJOA may cause fetal harm. The drug exposure window of approximately 690 days (based on 5 times the half-life of oteseconazole) precludes adequate mitigation of the embryo-fetal toxicity risks. Advise patients that VIVJOA is contraindicated in females of reproductive potential, and in pregnant and lactating women because of potential risks to a fetus or breastfed infant. ( 5.1, 8.1, 8.2, 8.3)
ADVERSE REACTIONS
The most frequently reported adverse reactions (incidence > 2%) were headache and nausea. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Mycovia Pharmaceuticals, Inc. at 1-855-299-0637 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
BCRP (Breast Cancer Resistance Protein) Substrates:Concomitant use of VIVJOA with BCRP substrates may increase the exposure of drugs that are BCRP substrates, which may increase the risk of adverse reactions associated with these drugs. Use the lowest possible starting dose of the BCRP substrate or consider reducing the dose of the substrate drugs and monitor for adverse reactions. ( 7.1)
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 4/2022
- There are two recommended VIVJOA dosage regimens: a VIVJOA-only regimen and a Fluconazole/VIVJOA regimen. Use one of these two dosage regimens. (
2.1)
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Vulvovaginal Candidiasis
1.2 Usage
2 DOSAGE AND ADMINISTRATION
2.1 Dosage Overview and Important Administration Instructions
2.2 VIVJOA-only Dosage Regimen
2.3 Fluconazole/VIVJOA Dosage Regimen
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Effect of VIVJOA on Other Drugs
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Vulvovaginal Candidiasis
VIVJOA is indicated to reduce the incidence of recurrent vulvovaginal candidiasis (RVVC) in females with a history of RVVC who are NOT of reproductive potential [see Warnings and Precautions (5.1), Use in Specific Populations (8.3), and Clinical Studies (14)] .
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosage Overview and Important Administration Instructions
There are two recommended VIVJOA dosage regimens: a VIVJOA-only regimen and a Fluconazole/ VIVJOA regimen. Use one of the following two dosage regimens:
- VIVJOA-only dosage regimen [see Dosage and Administration (2.2)]
- Fluconazole/VIVJOA dosage regimen [see Dosage and Administration (2.3)] .
Administer VIVJOA orally with food [see Clinical Pharmacology (12.3)]. Swallow the capsules whole. Do not chew, crush, dissolve, or open the capsules.
2.2 VIVJOA-only Dosage Regimen
For the VIVJOA-only dosage regimen:
- On Day 1:Administer VIVJOA 600 mg (as a single dose), then
- On Day 2:Administer VIVJOA 450 mg (as a single dose), then
- Beginning on Day 14:Administer VIVJOA 150 mg once a week (every 7 days) for 11 weeks (Weeks 2 through 12).
2.3 Fluconazole/VIVJOA Dosage Regimen
For the Fluconazole/VIVJOA dosage regimen, prescribe fluconazole and:
- On Day 1, Day 4, and Day 7:Administer fluconazole 150 mg orally, then
- On Days 14 through 20:Administer VIVJOA 150 mg once daily for 7 days, then
- Beginning on Day 28:Administer VIVJOA 150 mg once a week (every 7 days) for 11 weeks (Weeks 4 through 14).
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
VIVJOA is contraindicated in:
- Females of reproductive potential [see Warnings and Precautions (5.1)and Use in Specific Populations (8.3)]
- Pregnant and lactating women [see Warnings and Precautions (5.1), and Use in Specific Populations (8.1, 8.2)]
- Patients with known hypersensitivity to oteseconazole.
-
5 WARNINGS AND PRECAUTIONS
5.1 Embryo-Fetal Toxicity
VIVJOA is contraindicated in females of reproductive potential, and in pregnant and lactating women. Based on animal studies, VIVJOA may cause fetal harm. The drug exposure window of approximately 690 days (based on 5 times the half-life of oteseconazole) precludes adequate mitigation of the embryo-fetal toxicity risks. Ocular abnormalities were observed in the offspring of pregnant rats dosed at 7.5-mg/kg/day during organogenesis through lactation in pre and postnatal developmental studies. The observed ocular abnormalities included cataracts, opacities, exophthalmos/buphthalmos, optic nerve/retinal atrophy, lens degeneration and hemorrhage. Ocular abnormalities occurred at doses about 3.5 times the steady state clinical exposure seen with patients being treated for RVVC. Advise patients that VIVJOA is contraindicated in females of reproductive potential, and in pregnant and lactating women because of potential risks to a fetus or breastfed infant [see Use in Specific Populations (8.1, 8.2, 8.3)].
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of one drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
A total of 580 patients were treated with VIVJOA in three clinical trials (Trial 1, Trial 2, and Trial 3) [see Clinical Studies (14)] . Patients in the clinical trials were women with RVVC who received VIVJOA treatment for 12 weeks. The mean age of the patient population was 34 years (range:16-78 years), with 84% of patients aged 18-44 years and 16% of patients aged 45 years and older. Although females of reproductive potential were included in the clinical safety data, VIVJOA is contraindicated in females of reproductive potential due to the risk of embryo-fetal toxicity [see Contraindications (4), Warnings and Precautions (5.1), and Use in Specific Populations (8.1, 8.3, 8.4)] .
The clinical trials population was 75% (435/580) White, 17% (96/580) Black or African American, 6% (36/580) Asian, and 2% (13/580) Other women. Fifteen percent (86/580) of all women were Hispanic/Latino. Patients enrolled in the induction and maintenance phases of the clinical trials were treated with different VIVJOA dosage regimens versus comparators [see Clinical Studies (14)] .
The adverse reaction that led to discontinuation in 1 of 580 (0.2 %) VIVJOA-treated patients was allergic dermatitis. Overall, similar percentages of serious adverse reactions and adverse reactions leading to drug discontinuation were reported across the VIVJOA and comparator patient dosing groups.
The most frequently reported adverse reactions (incidence >2%) among VIVJOA-treated patients in Trial 1, Trial 2 and Trial 3 were headache (includes headache, migraines, sinus headaches) (7.4%) and nausea (3.6%).
Other Adverse Reactions
The following selected adverse reactions occurred in <2% of patients receiving VIVJOA in Trial 1, Trial 2 and Trial 3:
- Laboratory investigations:Increased blood creatine phosphokinase
- Gastrointestinal disorders:Dyspepsia
- Vascular disorders:Hot flush
- Renal and urinary disorders:Dysuria
- Reproductive system and breast disorders:Menorrhagia (includes genital hemorrhage, menorrhagia; menometrorrhagia; uterine hemorrhage, vaginal hemorrhage) metrorrhagia; vulvovaginal irritation (includes vulvovaginal burning sensation, vulvovaginal discomfort, and vulvovaginal pain)
Laboratory Findings
Elevations in Creatine Phosphokinase
Serum creatine phosphokinase (CPK) (an indirect marker of muscle injury/necrosis) elevations greater than or equal to 10 times the upper limit of normal were observed in 11 (1.9%) patients treated with VIVJOA versus 2 (0.7%) patients in the comparator groups during the VIVJOA clinical trials. The elevations were transient.
-
7 DRUG INTERACTIONS
7.1 Effect of VIVJOA on Other Drugs
BCRP (Breast Cancer Resistance Protein) Transporter Substrates
Oteseconazole is a BCRP inhibitor. Concomitant use of VIVJOA with BCRP substrates (e.g., rosuvastatin) may increase the exposure of BCRP substrates (e.g., rosuvastatin), which may increase the risk of adverse reactions associated with these drugs. Use the lowest possible starting dose of the BCRP substrate or consider reducing the dose of the substrate drug and monitor for adverse reactions [see Clinical Pharmacology (12.3)] .
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
VIVJOA is contraindicated in females of reproductive potential and in pregnant women. Based on animal studies, VIVJOA may cause fetal harm when administered to pregnant women. In addition, the drug exposure window of approximately 690 days (based on 5 times the half-life of oteseconazole) precludes adequate mitigation of the embryo-fetal toxicity risks [see Warnings and Precautions (5.1)and Clinical Pharmacology (12.3)].
Ocular abnormalities were observed in a pre and postnatal animal study in the offspring of rats administered oteseconazole from Gestation Day 6 through Lactation Day 20 at doses about 3.5 times the recommended human dose based on AUC comparisons (see Data). The observed ocular abnormalities included cataracts, opacities, exophthalmos/buphthalmos, optic nerve/retinal atrophy, lens degeneration and hemorrhage.
There are limited human data in pregnant women who were exposed to VIVJOA during the clinical trials; these data are insufficient to exclude a potential risk of cataracts or other eye abnormalities in human infants.
Data
Animal Data
Rat and rabbit embryofetal development was assessed after oral administration of oteseconazole. There was no embryofetal toxicity or malformations at 40 mg/kg/day following administration of oteseconazole during organogenesis in pregnant rats at doses about 10 times the maximum human exposure for RVVC based on AUC comparisons. Abortions occurred in rabbits in the presence of maternal toxicity (reduced bodyweight gain with reduced food consumption) but there were no malformations at 15 mg/kg/day following administration of oteseconazole during organogenesis in pregnant rabbits about 6 times the maximum human exposure for RVVC based on AUC comparisons.
Ocular abnormalities including cataracts, opacities, exophthalmos/buphthalmos, optic nerve/retinal atrophy, lens degeneration and hemorrhage were observed in the offspring of rats administered oteseconazole from Gestation Day 6 through Lactation Day 20 at 7.5 mg/kg day (about 3.5 times the recommended human dose based on AUC comparisons). There were no effects on pregnancy or parturition in these pre and postnatal studies at any dose.
8.2 Lactation
Risk Summary
VIVJOA is contraindicated in lactating women and females of reproductive potential. There are no data on the presence of oteseconazole in human or animal milk or data on the effects of oteseconazole on milk production. There were no reported adverse effects in breastfed infants following maternal exposure to oteseconazole during lactation; however, given the limited duration of follow-up of the oteseconazole-exposed infants during the post-natal period, no conclusions can be drawn from these data [see Warnings and Precautions (5.1)] .
Ocular abnormalities were observed in a pre and postnatal study in the offspring of rats administered oteseconazole from Gestation Day 6 through Lactation Day 20 at doses approximately 3.5 times the recommended human dose based on AUC comparisons [see Use in Specific Populations (8.1)]. The relationship between the observed animal findings and breastfed infants is unknown.
8.3 Females of Reproductive Potential
VIVJOA is contraindicated in females of reproductive potential based on animal findings. The drug exposure window of approximately 690 days (based on 5 times the half-life of oteseconazole) precludes adequate mitigation of the embryo-fetal toxicity risks [see Warnings and Precautions (5.1), Use in Specific Populations (8.1)and Clinical Pharmacology (12.3)].
Females who are NOT of reproductive potential are defined as: persons who are biological females who are postmenopausal or have another reason for permanent infertility (e.g., tubal ligation, hysterectomy, salpingo-oophorectomy).
8.4 Pediatric Use
VIVJOA is contraindicated in females of reproductive potential. Based on animal studies, VIVJOA may cause fetal harm when administered to a pregnant woman or potential harm to the breastfed infant. The drug exposure window of approximately 690 days (based on 5 times the half-life of oteseconazole) precludes adequate mitigation of the embryo-fetal toxicity risks associated with VIVJOA use [see Contraindications (4), Warnings and Precautions (5.1)and Use in Specific Populations (8.1, 8.2, 8.3)and Clinical Pharmacology (12.3)] .
The safety and effectiveness of VIVJOA have not been established in pre-menarchal pediatric females.
8.5 Geriatric Use
Clinical studies of VIVJOA did not include sufficient numbers of patients 65 years of age and older to determine whether they respond differently from younger adult patients.
8.6 Renal Impairment
No dosage adjustment of VIVJOA is recommended in patients with mild to moderate renal impairment (i.e., estimated glomerular filtration rate (eGFR) by the modification of diet in renal disease (MDRD) equation 30-89 mL/min). Clinical studies of VIVJOA did not include sufficient numbers of patients with severe renal impairment (eGFR 15-29 mL/min) or end-stage renal disease (ESRD), defined as eGFR <15 mL/min, to determine the safety of VIVJOA in this population. Therefore, VIVJOA is not recommended for use in patients with severe renal impairment or ESRD (with or without dialysis) [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
No dosage adjustment of VIVJOA is recommended in patients with mild hepatic impairment (Child-Pugh A). There is insufficient information to determine the safety of VIVJOA in patients with moderate or severe hepatic impairment (Child-Pugh B-C). Therefore, VIVJOA is not recommended for use in patients with moderate or severe hepatic impairment [see Clinical Pharmacology (12.3)].
-
11 DESCRIPTION
VIVJOA (oteseconazole capsules) contains oteseconazole which is an oral azole antifungal agent.
The chemical name of oteseconazole is ( R)-2-(2,4-difluorophenyl)-1,1-difluoro-3-(1 H-tetrazol-1-yl)-1-(5-(4-(2,2,2-trifluoroethoxy)phenyl)pyridin-2-yl)propan-2-ol or 2-Pyridineethanol, α-(2,4-difluorophenyl)-β β-difluoro- α-(1 H-tetrazol-1-ylmethyl)-5-(4-(2,2,2-trifluoroethoxy)phenyl)-,(α R)-. The empirical formula is C 23H 16F 7N 5O 2. The molecular weight is 527.39 g/mol. The structural formula is
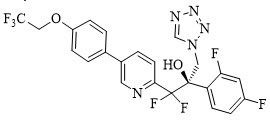
Oteseconazole is a white to off-white crystalline powder and is practically insoluble in water within a pH range of 1 to 9 but is soluble in a variety of organic solvents.
Each oteseconazole capsule, for oral use, contains 150 mg oteseconazole and the following inactive ingredients: croscarmellose sodium, hydroxypropyl cellulose, lactose, magnesium stearate, silicified microcrystalline cellulose, and sodium lauryl sulfate. Capsule shell and print constituents: FD&C Blue #1, FD&C Red #3, gelatin, Opacode SW-9008/SW-9009 and titanium dioxide. Contains no ingredient made from a gluten-containing grain (wheat, barley, or rye).
-
12 CLINICAL PHARMACOLOGY
12.2 Pharmacodynamics
Oteseconazole exposure-response relationships and the time course of pharmacodynamic response are unknown.
12.3 Pharmacokinetics
The AUC of oteseconazole increased approximately dose proportionally while the C maxincreased less than dose proportionally over a dose range of 20 mg (0.13 times the lowest recommended dose) to 320 mg (0.53 times the highest recommended dose). The pharmacokinetic parameters of oteseconazole associated with the administration of the recommended dosing regimen of VIVJOA are presented in Table 1.
Table 1: Pharmacokinetic (PK) Parameters of Oteseconazole PK Parameter * Mean (± SD) - * Following repeat dose administration of VIVJOA at the approved recommended dosage for RVVC at the end of treatment.
C max(µg/mL) 2.8 (1.25) AUC 24h(h∙µg/mL) 64.2 (29.4) C min(µg/mL) 2.5 (1.19) Distribution
The central volume of distribution of oteseconazole is approximately 423 L. Oteseconazole is 99.5-99.7% bound to plasma proteins. Animal studies indicated that oteseconazole exposures in vaginal tissue are comparable to plasma exposures.
Excretion
Following oral administration of radiolabeled oteseconazole, approximately 56% of the radiolabeled dose was recovered in feces primarily through biliary excretion and 26% was recovered in urine.
Specific Populations
There were no clinically significant differences in the pharmacokinetics of oteseconazole based on sex, race/ethnicity or mild to moderate renal impairment.
12.4 Microbiology
Mechanism of Action:
Oteseconazole is an azole metalloenzyme inhibitor targeting the fungal sterol, 14α demethylase (CYP51), an enzyme that catalyzes an early step in the biosynthetic pathway of ergosterol, a sterol required for fungal cell membrane formation and integrity. Inhibition of CYP51 results in the accumulation of 14-methylated sterols, some of which are toxic to fungi. Through the inclusion of a tetrazole metal-binding group, oteseconazole has a lower affinity for human CYP enzymes.
Resistance:
The potential for increases in minimum inhibitory concentrations (MIC) to oteseconazole has been evaluated in vitroincluding specific mechanisms of resistance .Increases in oteseconazole MIC were associated with upregulation of the efflux pumps CDR1, MDR1, and the azole target, lanosterol 14-alpha-demethylase (CYP51). Against certain Candidaspp. oteseconazole maintained meaningful in vitroactivity against clinical isolates that were resistant to fluconazole.
Antimicrobial Activity:
The following in vitrodata is available, but their clinical significance is unknown. Oteseconazole has been shown to be active against most isolates of the following microorganisms associated with RVVC [see Indications and Usage (1.1)] :
- Candida albicans
- Candida glabrata
- Candida krusei
- Candida parapsilosis
- Candida tropicalis
- Candida lusitaniae
- Candida dubliniensis
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
There was no increase in the incidence of tumors following daily oral administration of oteseconazole to Tg.rasH2 mice for 6 months at doses of 5 mg/kg/day (males) and 15 mg/kg/day (females), which are up to 3 and 10 times, respectively, the maximum human exposure for RVVC based on AUC comparisons.
In an oral carcinogenicity study, Sprague Dawley rats were administered doses of 0.5, 1.5, or 5 mg/kg/day oteseconazole once daily for up to 90 weeks. The high dose was initially reduced from 5 to 3 mg/kg/day due to excess mortality in males and reduced body weight in females. In males, an increase in the incidence in Leydig cell adenomas of the testes and thyroid follicular cell adenomas/carcinomas (combined) were increased at ≥1.5 mg/kg/day (similar to the maximum human exposure for RVVC based on AUC comparisons). In females, thyroid follicular cell adenomas and carcinomas (combined) were increased at ≥1.5 mg/kg/day (similar to the maximum human exposure for RVVC based on AUC comparisons) and thyroid carcinomas were increased at 5 to 3 mg/kg/day (5 times the maximum human exposure for RVVC based on AUC comparisons). The Leydig and thyroid findings are of uncertain relevance to humans.
Mutagenesis
Oteseconazole was negative in the bacterial reverse mutation assay, in vitrochromosomal aberration assay and micronucleus assay in rats.
Impairment of Fertility
Male rats were administered daily oral doses of 0, 0.5, 3, or 10 mg/kg/day oteseconazole beginning 42 days prior to pairing with untreated females, through the mating and post-mating period until euthanasia on Day 76 of treatment followed by a 12-week recovery period. There were no effects on reproductive and/or fertility parameters at the time of mating at 10 mg/kg/day (7 times the maximum human exposure for RVVC based on AUC comparisons). Increased incidences of abnormal sperm were observed at 3 mg/kg/day and sperm counts were reduced at 10 mg/kg/day. Although fertility was unaffected, sperm concentration remained reduced at the end of the recovery period.
Female rats were administered daily oral doses of 0, 1.5, 5, or 25 mg/kg/day oteseconazole beginning 28 days prior to cohabitation with untreated males, continuing throughout mating and through gestational day 7. Although there were no effects on estrous cyclicity, effects on reproductive and fertility parameters were observed at 25 mg/kg/day in the presence of maternal toxicity (11 times the maximum human exposure for RVVC based on AUC comparisons).
13.2 Animal Toxicology and/or Pharmacology
In an oral carcinogenicity study, Sprague Dawley rats were administered doses of 0.5, 1.5, or 5 mg/kg/day oteseconazole once daily for up to 90 weeks. The high dose was initially reduced from 5 to 3 mg/kg/day in males due to excess mortality. Incidences of hemorrhage were increased in the adrenals, brain, coagulating gland, ears, epididymides, head, heart, lung, nose, pancreas, pharynx, prostate, seminal vesicles, spinal cord, testes, thymus, and bladder of male Crl:CD ®(SD) rats (after 77 weeks of dosing at about 5 times the MRHD based on AUC comparisons). There were no increases in the incidence of hemorrhage in rats after 26 weeks at 5 mg/kg. The clinical relevance of these findings after very high doses (5 to 7 times the MRHD) for the lifetime of the rat remains unclear.
-
14 CLINICAL STUDIES
Overview of the Clinical Studies
A total of 656 adults and post-menarchal pediatric females with RVVC (defined as ≥3 episodes of vulvovaginal candidiasis (VVC) in a 12-month period) were randomized in two multicenter, multinational, double-blind, placebo-controlled trials: Trial 1 (NCT#03562156) and Trial 2 (NCT#03561701). A total of 219 adults and post-menarchal pediatric females with RVVC were randomized in a multicenter, double-blind trial [Trial 3 (NCT#03840616)]. Although females of reproductive potential were included in the clinical efficacy data, VIVJOA is contraindicated in females of reproductive potential due to the risk of embryo-fetal toxicity [see Contraindications (4), Warnings and Precautions (5.1)and Use in Specific Populations (8.1, 8.3, 8.4)] .
Trial 1 and Trial 2
Trial 1 and Trial 2 were both randomized, placebo-controlled trials evaluating the efficacy and safety of VIVJOA in the reduction of RVVC. Both trials consisted of two phases: an open-label induction phase and an 11-week maintenance phase. Patients received three sequential doses of 150 mg of fluconazole (every 72 hours) on Days, 1, 4 and 7 during the induction phase. Patients returned 14 days after the first dose of fluconazole and if the acute VVC episode was resolved (vulvovaginal signs and symptoms score < 3) they were randomized (2:1) to receive either 150 mg of VIVJOA or placebo for 7 days followed by 11 weekly doses in the maintenance phase.
In Trial 1, a total of 483 patients were enrolled in the induction phase with 326 patients entering the maintenance phase with 217 patients randomized to VIVJOA and 109 patients randomized to placebo. A total of 182 patients (84%) in the VIVJOA group and 91 patients (83%) in the placebo group completed the trial. The mean age of patients was 34 years old (range 17-78 years old) with 85% of patients aged 18-44 years and 15% of patients aged 45 years and older. Patients were 72% White, 13% Black or African American, 14% Asian, and 8% were of Hispanic or Latino ethnicity.
In Trial 2, a total of 425 patients were enrolled into the induction phase with 330 patients entering the maintenance phase with 220 subjects randomized to VIVJOA and 110 patients randomized to placebo. A total of 191 patients (87%) in the VIVJOA group and 91 patients (83%) in the placebo group completed the trial. The mean age of patients was 34 years old (range 18-73 years old) with 85% of patients aged 18-44 years and 15% of patients aged 45 years and older. Patients were 89% White, 10% Black or African American and 15% were of Hispanic or Latino ethnicity.
For both Trial 1 and Trial 2, efficacy was assessed by the proportion of patients with ≥1 culture-verified acute VVC episode (positive fungal culture for Candidaspecies associated with a clinical signs and symptoms score of ≥3) during the Maintenance Phase through Week 48. Evaluation of clinical signs and symptoms included erythema (redness), edema (swelling), excoriation (skin picking), itching, burning and irritation. Since treatment for acute VVC was allowed to be provided to a patient if it was deemed to be clinically needed when the patient had a signs and symptoms score ≥ 3 and a positive KOH test, the proportion of patients with ≥1 culture-verified acute VVC episode or who took medication known to treat VVC during the Maintenance Phase through Week 48 is also presented.
VIVJOA was superior to placebo with reference to the proportion of patients with ≥1 culture-verified acute VVC episode through Week 48 (Table 2) or the proportion of patients with ≥1 culture-verified acute VVC episode or who took medication known to treat VVC during the Maintenance Phase through Week 48. For both Trial 1 and Trial 2, the average percentage of patients was lower in the VIVJOA groups compared with the placebo group (Table 2).
Table 2: Trial 1 and 2 Efficacy Endpoints: ITT Population Trial 1 Trial 2 VIVJOA
(N=217)Placebo
(N=109)VIVJOA
(N=218)Placebo
(N=108)Abbreviations: ITT=Intent-to-Treat (Population); VVC=vulvovaginal candidiasis. - * Average %. Missing values were imputed with multiple imputation using the following auxiliary information: region, treatment, Baseline body mass index, Baseline age, ethnicity, and visit.
- † The p-value was obtained using a Chi-square test comparing VIVJOA with placebo.
Proportion of Patients with ≥1 Culture-verified Acute VVC Episode (Day 1 through Week 48) * 6.7% 42.8% 3.9% 39.4% Treatment Difference p-value † <0.001 <0.001 Proportion of Patients with ≥1 Culture-verified Acute VVC Episode or received VVC medication (Day 1 through Week 48) * 27.3% 50.8% 21.3% 49.7% Treatment Difference p-value † <0.001 <0.001 Trial 3
Trial 3 was a randomized, double-blind trial evaluating the efficacy and safety of VIVJOA versus fluconazole and placebo in adults and post-menarchal pediatric females with RVVC. The trial consisted of two phases: induction and maintenance.
During the induction phase, patients received 1050 mg of VIVJOA over two days (600 mg [4×150mg] on Day 1 and 450 mg [3×150mg] on Day 2) or three sequential doses of 150 mg of fluconazole (every 72 hours) on Days, 1, 4 and 7. Patients returned 14 days after the first dose and moved to the maintenance phase if the acute VVC episode was resolved. During the maintenance phase, patients received 150 mg VIVJOA weekly or placebo weekly for 11 weeks.
A total of 219 patients were randomized (2:1) into the induction phase: 147 to VIVJOA and 72 to fluconazole/placebo. One patient in the VIVJOA group did not receive drug therefore 146 patients received VIVJOA. A total of 112 patients (76%) in the VIVJOA group and 55 patients (76%) in the fluconazole/placebo group completed the trial.
The mean age of patients was 35 years (range 16-78) with 80% of patients aged 18-44 years and 19% of patients aged 45 years and older. Patients were 59% White, 34% Black or African American, 1% Asian and 26% were of Hispanic or Latino ethnicity. The trial was conducted completely in the United States.
Efficacy was assessed by the proportion of patients with ≥1 culture verified acute VVC episode during the maintenance phase (post-randomization through Week 50) or who failed clearing their infection during the induction phase. A recurring acute VVC episode was defined as a positive culture for Candidaspecies and a clinical signs and symptoms score of ≥3. Evaluation of clinical signs and symptoms included erythema(redness), edema (swelling), excoriation (skin picking), itching, burning and irritation. Additionally, the proportion of patients with ≥1 culture verified acute VVC episode or who took medication known to treat VVC during the maintenance phase (post-randomization through Week 50) or who failed clearing their infection during the induction phase is presented.
VIVJOA was superior to fluconazole/placebo in the proportion of patients with ≥1 culture-verified recurring acute VVC episode during the maintenance phase (post randomization through Week 50) or failed clearing their infection during the induction phase and the proportion of patients with ≥1 culture-verified recurring acute VVC episode or took VVC medication known to treat VVC during the maintenance phase (post randomization through Week 50) or who failed clearing their infection during the induction phase. The average percentage of patients was lower in the VIVJOA group compared with the fluconazole/placebo group (Table 3).
Table 3: Trial 3 Efficacy Endpoints ITT population VIVJOA
(N=147)Fluconazole/Placebo
(N=72)Treatment Difference p-value * Abbreviations: ITT=Intent-to-Treat (Population); VVC=vulvovaginal candidiasis - * The p-value was obtained using a Chi-square test comparing VIVJOA with fluconazole/placebo.
- † Average %, Missing values were imputed with multiple imputation using the following auxiliary information: treatment, baseline body mass index, baseline age, ethnicity, and visit.
Proportion of Patients with ≥1 Culture-verified Acute VVC Episode through Week 50 or Unresolved VVC Episode During the Induction Phase † 10.3% 42.9% <0.001 Proportion of Patients with ≥1 Culture-verified Acute VVC Episode or took VVC medication through Week 50 or Unresolved VVC Episode During the Induction Phase † 43.5% 59.0% 0.039 -
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
VIVJOA (oteseconazole capsules) are supplied as lavender hard gelatin capsules. Printed black "OTE 150" on the capsule and contain 150 mg oteseconazole. They are available in an 18-count (NDC: 74695-823-18) blister package within a child resistant wallet. There will be one blister pack per wallet and one wallet per outer carton.
The fluconazole/VIVJOA dosage regimen is in an 18-count (NDC: 74695-945-18) blister package within a child resistant wallet. There is one blister pack of VIVJOA (oteseconazole capsules) per wallet and one wallet per outer carton. The outer carton and wallet contain the following: "fluconazole/VIVJOA dosage regimen" and "fluconazole is prescribed separately".
Fluconazole is not supplied in the carton.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Embryo-Fetal Toxicity
Advise patients that VIVJOA is contraindicated in females of reproductive potential and in pregnant women because it may cause fetal harm [see Contraindications (4), Warnings and Precautions (5.1)and Use in Specific Populations (8.1, 8.3)] .
Lactation
Advise patients that VIVJOA is contraindicated in lactating women because it may cause harm to the breastfed infant [see Contraindications (4), Warnings and Precautions (5.1)and Use in Specific Populations (8.2)].
Important Administration Instructions
Advise patients that VIVJOA must be taken with food, and that capsules must be swallowed whole and not chewed, crushed, dissolved, or opened [see Dosage and Administration (2.1)].
Concomitant Administration with BCRP Transporter Substrates
Advise patients to inform their health care provider if they are taking a BCRP substrate (e.g., rosuvastatin). Concomitant use with VIVJOA may increase the exposure of drugs that are BCRP substrates, which may increase the risk of adverse reactions associated with these drugs [see Drug Interactions (7.1)] .
- SPL UNCLASSIFIED SECTION
-
PATIENT PACKAGE INSERT
PATIENT INFORMATION
VIVJOA™ [viv joe ah]
(oteseconazole)
capsules, for oral useThis Patient Information has been approved by the U.S. Food and Drug Administration. Approved: X/2022 What is VIVJOA? - VIVJOA is a prescription medicine used to reduce the risk of vaginal yeast infections that keep coming back (recurrent vulvovaginal candidiasis, RVVC) in females with a history of RVVC.
- VIVJOA should only be taken by females who are not pregnant and who are not able to become pregnant.
It is not known if VIVJOA is safe and effective in females who have not had their first menstrual period. - Are able to become pregnant.
- Are pregnant or plan to become pregnant. VIVJOA may harm your unborn baby. Tell your doctor if you are pregnant, think you might be pregnant, or plan to become pregnant.
- Are breastfeeding or plan to breastfeed. It is not known if VIVJOA passes into your breastmilk.
- Are allergic to oteseconazole or any of the ingredients in VIVJOA. See the end of this Patient Information leaflet for a complete list of ingredients in VIVJOA.
Before you take VIVJOA, tell your doctor about all of your medical conditions, including if you: - have kidney problems
- have liver problems
Tell your doctor about all the medicines you take,including prescription and over-the-counter medicines, vitamins, and herbal supplements. Especially tell your doctor if you take medicines called breast cancer resistance protein (BCRP) transporter substrates, including the cholesterol lowering medicine rosuvastatin. Ask your doctor or pharmacist for a list of these medicines if you are not sure. Taking VIVJOA (a BCRP inhibitor) with BCRP transporter substrates may increase the risk of side effects with these medicines. Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine. How should I take VIVJOA? -
Your doctor has prescribed VIVJOA:
- Take VIVJOA exactly as your doctor tells you to take it. Talk to your doctor if you have any questions.
- On Day 1:Take VIVJOA 600 mg(four capsules as a single dose), by mouth then,
- On Day 2:Take VIVJOA 450 mg(three capsules as a single dose), by mouth then,
- On Day 14: Take VIVJOA 150 mg(one capsule) one time a week(every 7 days) by mouth for 11 weeks.
- Take VIVJOA capsules by mouth with food.
- Swallow VIVJOA capsules whole. Do not chew, crush, dissolve, or open the capsules.
What are the possible side effects of VIVJOA? - See " Do not take VIVJOA if you:" Females who are pregnant, are able to become pregnant, and females who are breastfeeding should not take VIVJOA.
The most common side effects of VIVJOA areheadache and nausea. These are not all the possible side effects of VIVJOA. For more information, ask your doctor or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. How should I store VIVJOA? - Store VIVJOA at room temperature between 68°F to 77°F (20°C to 25°C).
- Protect from light when removed from the outer carton.
Keep VIVJOA and all medicines out of the reach of children. General information about the safe and effective use of VIVJOA. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use VIVJOA for a condition for which it has not been prescribed. Do not give VIVJOA to other people, even if they have the same symptoms that you have. It may harm them. You can ask your doctor or pharmacist for information about VIVJOA that is written for health professionals. What are the ingredients in VIVJOA? Active Ingredient:oteseconazole Inactive Ingredients:croscarmellose sodium, hydroxypropyl cellulose, lactose, magnesium stearate, silicified microcrystalline cellulose, and sodium lauryl sulfate. Capsule shell and print constituents: FD&C Blue #1, FD&C Red #3, gelatin, Opacode SW-9008/SW-9009 and titanium dioxide. VIVJOA does not contain gluten (wheat, barley, or rye). Manufactured for and Distributed by:
Mycovia Pharmaceuticals, Inc.
Durham, NC 27703
© 2021 Mycovia Pharmaceuticals, Inc. All rights reserved.
For more information, go to www.mycovia.comor call 1-855-299-0637. -
PRINCIPAL DISPLAY PANEL - 150 mg Capsule Blister Pack Label
Dosing directions for VIVJOA™ (oteseconazole capsules), 150 mg per capsule, for oral use
▼ START HERE
DAY
1
DAY
1
DAY
1
DAY
1DAY 1: Take ALL FOUR capsules together.
DAY
2
DAY
2
DAY
2DAY 2: Take ALL THREE capsules together.
WEEK
2
WEEK
3
WEEK
4Beginning on DAY 14, take ONE capsule weekly.
WEEK
5
WEEK
6
WEEK
7To remove capsule, press from the reverse side.
WEEK
8
WEEK
9
WEEK
10Manufactured for Mycovia Pharmaceuticals, Inc. by Patheon.
WEEK
11
WEEK
12Distributed by Mycovia Pharmaceuticals, Inc.
Rx ONLY
LOT XXXXX0000
EXP MMM YYYY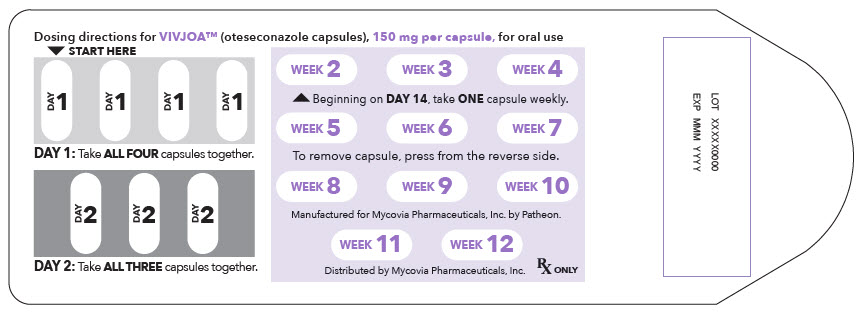
-
PRINCIPAL DISPLAY PANEL - 150 mg Capsule Blister Pack Container
NDC: 74695-823-18
Rx only18 CAPSULES
Press and
hold button.vivjoa™
(oteseconazole capsules)
150 mg per capsule, for oral useSee package insert for full Prescribing Information.
STEP 1 Press and hold button.
STEP 2 Pull out medication card.
Pull out here.
▼
-
PRINCIPAL DISPLAY PANEL - 150 mg Capsule Blister Pack Container Carton
NDC: 74695-823-18
Rx onlySee package insert for full Prescribing Information.
vivjoa™
(oteseconazole capsules)150 mg per capsule, for oral use
18 CAPSULES

-
INGREDIENTS AND APPEARANCE
VIVJOA
oteseconazole capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 74695-823 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OTESECONAZOLE (UNII: VHH774W97N) (OTESECONAZOLE - UNII:VHH774W97N) OTESECONAZOLE 150 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) 20 mg SILICON DIOXIDE (UNII: ETJ7Z6XBU4) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYDROXYPROPYL CELLULOSE (90000 WAMW) (UNII: UKE75GEA7F) SODIUM LAURYL SULFATE (UNII: 368GB5141J) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color purple (lavendar opaque) Score no score Shape CAPSULE Size 2mm Flavor Imprint Code OTE;150 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 74695-823-18 1 in 1 CARTON 07/11/2022 1 1 in 1 CONTAINER 1 18 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA215888 07/11/2022 Labeler - Mycovia Pharmaceuticals, Inc. (079575708) Establishment Name Address ID/FEI Business Operations Patheon, Inc. (a ThermoFischer Company) 205475333 manufacture(74695-823) Establishment Name Address ID/FEI Business Operations Patheon, Inc. 240769596 analysis(74695-823) Establishment Name Address ID/FEI Business Operations Packaging Coordinators, LLC. 078525133 pack(74695-823) , label(74695-823) Establishment Name Address ID/FEI Business Operations Evonik Corporation Tippecanoe Laboratories 130890994 api manufacture(74695-823) Establishment Name Address ID/FEI Business Operations Micro-macinazione SA Lonza Pharm and Biotech 480918515 particle size reduction(74695-823) Establishment Name Address ID/FEI Business Operations Dynamit Nobel GmBH Explosivoff-und Systemtechnik (Novasep) 313113144 api manufacture(74695-823) Establishment Name Address ID/FEI Business Operations ChemCon 328823245 analysis(74695-823) Establishment Name Address ID/FEI Business Operations Currenta GmBH &Co OHG 331575303 analysis(74695-823) Establishment Name Address ID/FEI Business Operations BioChem Labor fur biologische und chemische Analytik GmBH 318354230 analysis(74695-823)
Trademark Results [VIVJOA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 VIVJOA 88831981 not registered Live/Pending |
Mycovia Pharmaceuticals, Inc. 2020-03-12 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.