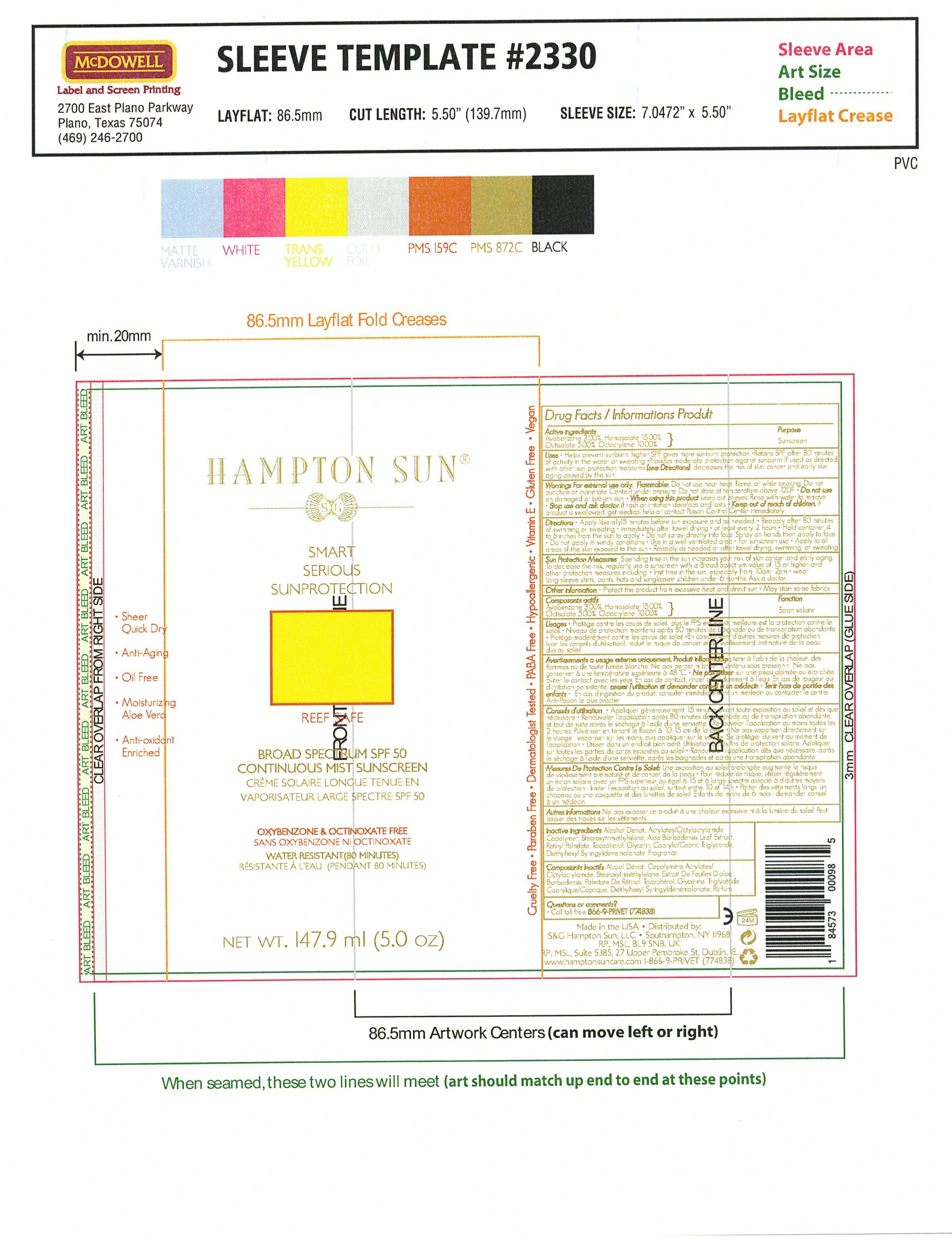
Hampton Sun SPF 50 Sunscreen Spry
Hampton Sun SPF 50 by
Drug Labeling and Warnings
Hampton Sun SPF 50 by is a Otc medication manufactured, distributed, or labeled by Inspec Solutions. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
HAMPTON SUN SPF 50- avobenzone, humosalate, octisalate, octocrylene spray
Inspec Solutions
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Hampton Sun SPF 50 Sunscreen Spry
When using this product:
keep out of eyes. Rinse with water to remove.
Keep away from face to avoid breathing it.
Do not puncture or incinerate, Contents under pressure
Do not store at temperature above 120⁰F
Stop use and ask a doctor if rash occurs.
Avobenzone 3.0% ……………………………………………………………………………… Sunscreen
Homosalate 15.0% ……………………………………………………………………………. Sunscreen
Octisalate 5.0% …………………………………………………………………………………. Sunscreen
Octocrylene 10.0% ……………………………………………………………………………. Sunscreen
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Behenyl Alcohol
BHT
Butyloctyl Salicylate
Caprylyl Methicone Dimethicone
Dimethyl Capramide
Disodium EDTA
Ethylhexyl Stearate
Ethylhexylglycerin
Fragrance
Glyceryl Stearate Hydrated Silica
PEG-100 Stearate
Phenoxyethanol
Polyester-8
Sodium Polyacrylate
Styrene/Acrylates Copolymer
Trideceth-6
Trimethylsiloxysilicate
VP/Hexadecene Copolymer
Water
Xanthan Gum
| HAMPTON SUN SPF 50
avobenzone, humosalate, octisalate, octocrylene spray |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Inspec Solutions (081030372) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Inspec Solutions | 081030372 | manufacture(72667-030) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.