These highlights do not include all the information needed to use FORTICAL® safely and effectively. See full prescribing information for FORTICAL. FORTICAL® (calcitonin-salmon [rDNA origin]) Nasal Spray for intranasal useInitial U.S. Approval: 2005
Fortical by
Drug Labeling and Warnings
Fortical by is a Prescription medication manufactured, distributed, or labeled by Upsher-Smith Laboratories, Inc., Enteris BioPharma, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
FORTICAL- calcitonin salmon spray, metered
Upsher-Smith Laboratories, Inc.
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use FORTICAL® safely and effectively. See full prescribing information for FORTICAL.
FORTICAL® (calcitonin-salmon [rDNA origin]) Nasal Spray for intranasal use Initial U.S. Approval: 2005 INDICATIONS AND USAGEFortical is a calcitonin indicated for the treatment of postmenopausal osteoporosis in women greater than 5 years postmenopause when alternative treatments are not suitable. Fracture reduction efficacy has not been demonstrated (1.1). Limitations of Use: DOSAGE AND ADMINISTRATIONDOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONSThe most common adverse reactions (3% or greater) are rhinitis, epistaxis and other nasal symptoms, back pain, arthralgia, and headache. (6) DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONSSee 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling. Revised: 8/2019 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.1 Treatment of Postmenopausal Osteoporosis
Fortical nasal spray is indicated for the treatment of postmenopausal osteoporosis in women greater than 5 years postmenopause. Fracture reduction efficacy has not been demonstrated. Fortical nasal spray should be reserved for patients for whom alternative treatments are not suitable (e.g., patients for whom other therapies are contraindicated or for patients who are intolerant or unwilling to use other therapies).
1.2 Important Limitations of Use
- Due to the possible association between malignancy and calcitonin-salmon use, the need for continued therapy should be re-evaluated on a periodic basis [see Warnings and Precautions (5.4)].
- Calcitonin-salmon nasal spray has not been shown to increase spinal bone mineral density in early postmenopausal women.
2 DOSAGE AND ADMINISTRATION
2.1 Basic Dosing Information
The recommended dose of Fortical nasal spray is 1 spray (200 International Units) per day intranasally, alternating nostrils daily.
2.2 Priming (Activation) of Pump
Unopened Fortical nasal spray should be stored in the refrigerator. Before using the first dose of Fortical nasal spray, the patient should wait until the bottle has reached room temperature. Remove the protective cap and clip from the bottle of Fortical nasal spray. To prime the pump before it is used for the first time, the bottle should be held upright and the two white side arms of the pump depressed toward the bottle at least 5 times until a full spray is produced. The pump is primed once the first full spray is emitted. To administer, the nozzle should be carefully placed into the nostril with the patient's head in the upright position, then the pump should be firmly depressed toward the bottle. The pump should not be primed before each daily use.
3 DOSAGE FORMS AND STRENGTHS
Fortical nasal spray consists of one glass bottle and one screw-on pump. The bottle contains 3.7 mL of calcitonin-salmon clear solution at a concentration of 2200 International Units per mL. A primed pump delivers 0.09 mL (200 International Units) calcitonin-salmon per actuation.
4 CONTRAINDICATIONS
Hypersensitivity to calcitonin-salmon or any of the excipients. Reactions have included anaphylactic shock, anaphylaxis, bronchospasm, and swelling of the tongue or throat [see Warnings and Precautions (5.1)].
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Serious hypersensitivity reactions have been reported in patients receiving calcitonin-salmon nasal spray, e.g., bronchospasm, swelling of the tongue or throat, anaphylaxis and anaphylactic shock. Reports of serious hypersensitivity reactions with injectable calcitonin-salmon have also been reported, including reports of death attributed to anaphylaxis. The usual provisions should be made for emergency treatment if such a reaction occurs. Hypersensitivity reactions should be differentiated from generalized flushing and hypotension [see Contraindications (4)].
For patients with suspected hypersensitivity to calcitonin-salmon, skin testing should be considered prior to treatment utilizing a dilute, sterile solution of a calcitonin-salmon injectable product. Healthcare providers may wish to refer patients who require skin testing to an allergist. A detailed skin testing protocol is available from Upsher-Smith Laboratories, Inc. by calling toll-free at 1-888-650-3789.
5.2 Hypocalcemia
Hypocalcemia associated with tetany (i.e. muscle cramps, twitching) and seizure activity has been reported with calcitonin therapy. Hypocalcemia must be corrected before initiating therapy with Fortical nasal spray. Other disorders affecting mineral metabolism (such as vitamin D deficiency) should also be effectively treated. In patients with these conditions, serum calcium and symptoms of hypocalcemia should be monitored during therapy with Fortical nasal spray. Use of Fortical nasal spray is recommended in conjunction with an adequate intake of calcium and vitamin D [see Dosage and Administration (2.3)].
5.3 Nasal Adverse Reactions
Adverse reactions related to the nose including rhinitis and epistaxis have been reported. Development of mucosal alterations may occur. Therefore, periodic nasal examinations with visualization of the nasal mucosa, turbinates, septum and mucosal blood vessels are recommended prior to start of treatment with Fortical nasal spray, periodically during the course of therapy, and at any time nasal symptoms occur.
Fortical nasal spray should be discontinued if severe ulceration of the nasal mucosa occurs, as indicated by ulcers greater than 1.5 mm in diameter or penetrating below the mucosa, or those associated with heavy bleeding. Although smaller ulcers often heal without withdrawal of Fortical nasal spray, medication should be discontinued temporarily until healing occurs [see Adverse Reactions (6.1)].
5.4 Malignancy
In a meta-analysis of 21 randomized, controlled clinical trials with calcitonin-salmon (nasal spray or investigational oral formulations), the overall incidence of malignancies reported was higher among calcitonin-salmon-treated patients (4.1%) compared with placebo-treated patients (2.9%). This suggests an increased risk of malignancies in calcitonin-salmon-treated patients compared to placebo-treated patients. The benefits for the individual patient should be carefully considered against possible risks [see Adverse Reactions (6.1)].
5.5 Antibody Formation
Circulating antibodies to calcitonin-salmon have been reported with calcitonin-salmon nasal spray. The possibility of antibody formation should be considered in any patient with an initial response to Fortical nasal spray who later stops responding to treatment [see Adverse Reactions (6.3)].
5.6 Urine Sediment Abnormalities
Coarse granular casts and casts containing renal tubular epithelial cells were reported in young adult volunteers at bed rest who were given injectable calcitonin-salmon to study the effect of immobilization on osteoporosis. There was no other evidence of renal abnormality and the urine sediment normalized after calcitonin-salmon was stopped. Periodic examinations of urine sediment should be considered. Urine sediment abnormalities have not been reported in ambulatory volunteers treated with calcitonin-salmon nasal spray.
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed in greater detail in other sections of the label:
- Hypersensitivity Reactions, including anaphylaxis [see Warnings and Precautions (5.1)]
- Hypocalcemia [see Warnings and Precautions (5.2)]
- Nasal Adverse Reactions [see Warnings and Precautions (5.3)]
- Malignancy [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of calcitonin-salmon nasal spray in the treatment of postmenopausal osteoporosis was assessed in 5 randomized, double-blind, placebo controlled trials that enrolled postmenopausal women, aged 45-75 years. The duration of the trials ranged from 1 to 2 years. The incidence of adverse reactions reported in studies involving postmenopausal osteoporotic patients chronically exposed to calcitonin-salmon nasal spray (N=341) and to placebo nasal spray (N=131), and reported in greater than 3% of calcitonin-salmon treated patients are presented in the following table. Other than flushing, nausea, possible allergic reactions, and possible local irritative effects in the respiratory tract, a relationship to calcitonin-salmon nasal spray has not been established.
| Adverse Reaction | Calcitonin-Salmon Nasal Spray N=341 % of Patients | Placebo Nasal Spray N=131 % of Patients |
|---|---|---|
|
|
||
| Rhinitis | 12 | 7 |
| Symptom of Nose* | 11 | 16 |
| Back Pain | 5 | 2 |
| Arthralgia | 4 | 5 |
| Epistaxis | 4 | 5 |
| Headache | 3 | 5 |
Nasal Adverse Reactions: In all postmenopausal patients treated with calcitonin-salmon nasal spray, the most commonly reported nasal adverse reactions included rhinitis (12%), epistaxis (4%), and sinusitis (2%). Smoking did not have a contributory effect on the occurrence of nasal adverse reactions.
Adverse reactions reported in 1-3% of patients treated with calcitonin-salmon nasal spray include: influenza-like symptoms, erythematous rash, arthrosis, myalgia, sinusitis, upper respiratory tract infection, bronchospasm, abdominal pain, nausea, dizziness, paresthesia, abnormal lacrimation, conjunctivitis, lymphadenopathy, infection, and depression.
Malignancy
A meta-analysis of 21 randomized, controlled clinical trials with calcitonin-salmon (nasal spray or investigational oral formulations) was conducted to assess the risk of malignancies in calcitonin-salmon-treated patients compared to placebo-treated patients. The trials in the meta-analysis ranged in duration from 6 months to 5 years and included a total of 10883 patients (6151 treated with calcitonin-salmon and 4732 treated with placebo). The overall incidence of malignancies reported in these 21 trials was higher among calcitonin-salmon-treated patients (254/6151 or 4.1%) compared with placebo-treated patients (137/4732 or 2.9%). Findings were similar when analyses were restricted to the 18 nasal spray only trials [calcitonin-salmon 122/2712 (4.5%); placebo 30/1309 (2.3%)].
The meta-analysis results suggest an increased risk of overall malignancies in calcitonin-salmon-treated patients compared to placebo-treated patients when all 21 trials are included and when the analysis is restricted to the 18 nasal spray only trials (see Table 2). It is not possible to exclude an increased risk when calcitonin-salmon is administered by the subcutaneous, intramuscular, or intravenous route because these routes of administration were not investigated in the meta-analysis. The increased malignancy risk seen with the meta-analysis was heavily influenced by a single large 5-year trial, which had an observed risk difference of 3.4% [95% CI (0.4%, 6.5%)]. Imbalances in risks were still observed when analyses excluded basal cell carcinoma (see Table 2); the data were not sufficient for further analyses by type of malignancy. A mechanism for these observations has not been identified. Although a definitive causal relationship between calcitonin-salmon use and malignancies cannot be established from this meta-analysis, the benefits for the individual patient should be carefully evaluated against all possible risks [see Warnings and Precautions (5.4)].
| Patients | Malignancies | Risk Difference* (%) | 95% Confidence Interval† (%) |
|---|---|---|---|
|
|
|||
| All (nasal spray + oral) | All | 1.0 | (0.3, 1.6) |
| All (nasal spray + oral) | Excluding basal cell carcinoma | 0.5 | (-0.1, 1.2) |
| All (nasal spray only) | All | 1.4 | (0.3, 2.6) |
| All (nasal spray only) | Excluding basal cell carcinoma | 0.8 | (-0.2, 1.8) |
6.2 Postmarketing Experience
Because postmarketing adverse reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The following adverse reactions have been reported during post-approval use of calcitonin-salmon nasal spray.
Allergic / Hypersensitivity Reactions: Serious allergic reactions have been reported in patients receiving calcitonin-salmon nasal spray, including anaphylaxis and anaphylactic shock.
Hypocalcemia: Hypocalcemia with paresthesia has been reported.
Body as a whole: facial or peripheral edema
Cardiovascular: hypertension, vasodilatation, syncope, chest pain
Nervous system: dizziness, seizure, visual or hearing impairment, tinnitus
Respiratory/ Special Senses: cough, bronchospasm, dyspnea, loss of taste/smell
Skin: rash/dermatitis, pruritus, alopecia, increased sweating
Gastrointestinal: diarrhea
Nervous system disorders: tremor
6.3 Immunogenicity
Consistent with the potentially immunogenic properties of medicinal products containing peptides, administration of Fortical may trigger the development of anti-calcitonin antibodies. In a two-year calcitonin-salmon nasal spray clinical study that evaluated immunogenicity, a measurable antibody titer was found in 69% of patients treated with calcitonin-salmon and 3% of placebo-treated patients. Antibody formation may be associated with a loss of response to treatment [see Warnings and Precautions (5.5)].
The incidence of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of a positive antibody test result may be influenced by several factors, including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of antibodies to calcitonin-salmon nasal spray with the incidence of antibodies to other calcitonin-containing products may be misleading.
7 DRUG INTERACTIONS
No formal drug interaction studies have been performed with calcitonin-salmon nasal spray.
Concomitant use of calcitonin-salmon and lithium may lead to a reduction in plasma lithium concentrations due to increased urinary clearance of lithium. The dose of lithium may require adjustment.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C:
Risk Summary
There are no adequate and well-controlled studies in pregnant women. Fortical nasal spray should be used during pregnancy only if the potential benefit justifies the use as compared with potential risks to the patient and fetus. Based on animal data, Fortical is predicted to have low probability of increasing the risk of adverse developmental outcomes above background risk.
Animal Data
Synthetic calcitonin-salmon has been shown to cause a decrease in fetal birth weights in rabbits when given by subcutaneous injection at doses 70-278 times the intranasal dose recommended for human use based on body surface area.
No embryo/fetal toxicities related to synthetic calcitonin-salmon were reported from maternal subcutaneous daily doses in rats up to 80 International Units/kg/day from gestation day 6 to 15.
8.3 Nursing Mothers
It is not known whether this drug is excreted in human milk. No studies have been conducted to assess the impact of Fortical on milk production in humans, its presence in human breast milk, or its effects on the breast-fed child. Because many drugs are excreted in human milk, caution should be exercised when Fortical is administered to a nursing woman. Synthetic calcitonin-salmon has been shown to inhibit lactation in rats.
8.5 Geriatric Use
In a multi-centered, double-blind, randomized clinical study of calcitonin-salmon nasal spray, 279 patients were less than 65 years old, while 467 patients were 65 to 74 years old and 196 patients were 75 and over. Compared to subjects less than 65 years old, the incidence of nasal adverse reactions (rhinitis, irritation, erythema, and excoriation) was higher in patients over the age of 65, particularly those over the age of 75. Other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
10 OVERDOSAGE
The pharmacologic actions of Fortical nasal spray suggest that hypocalcemic tetany could occur in overdose. Therefore, provisions for parenteral administration of calcium should be available for the treatment of overdose.
Single doses of calcitonin-salmon nasal spray up to 1600 International Units, doses up to 800 International Units per day for 3 days and chronic administration of doses up to 600 International Units per day have been studied without serious adverse effects.
11 DESCRIPTION
Calcitonin is a polypeptide hormone secreted by the parafollicular cells of the thyroid gland in mammals and by the ultimobranchial gland of birds and fish.
The active ingredient in Fortical (calcitonin-salmon [rDNA origin]) nasal spray is a polypeptide of 32 amino acids manufactured by recombinant DNA technology and is identical to calcitonin-salmon produced by chemical synthesis.
This is shown by the following graphic formula:

It is provided in a 3.7 mL fill glass bottle as a solution for intranasal administration with sufficient medication for at least 30 doses. Each spray delivers 200 International Units calcitonin-salmon in a volume of 0.09 mL.
Active Ingredient: Calcitonin-salmon 2200 International Units/mL, corresponding to 200 International Units per actuation (0.09 mL).
Inactive Ingredients: Sodium Chloride, Citric Acid, Phenylethyl Alcohol, Benzyl Alcohol, Polysorbate 80, Hydrochloric Acid or Sodium Hydroxide (added as necessary to adjust pH) and Purified Water.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Calcitonin-salmon is a calcitonin receptor agonist. Calcitonin-salmon acts primarily on bone, but direct renal effects and actions on the gastrointestinal tract are also recognized. Calcitonin-salmon appears to have actions essentially identical to calcitonins of mammalian origin, but its potency per mg is greater and it has a longer duration of action.
The actions of calcitonin on bone and its role in normal human bone physiology are still not completely elucidated, although calcitonin receptors have been discovered in osteoclasts and osteoblasts.
12.2 Pharmacodynamics
The information below, describing the clinical pharmacology of calcitonin, has been derived from studies with injectable calcitonin-salmon. The mean bioavailability of calcitonin-salmon nasal spray is approximately 3% of the injectable calcitonin-salmon in healthy subjects and, therefore, the conclusions concerning the clinical pharmacology of this preparation may be different.
Bone
Single injections of calcitonin-salmon caused a marked transient inhibition of the ongoing bone resorptive process. With prolonged use, there is a persistent, smaller decrease in the rate of bone resorption. Histologically, this is associated with a decreased number of osteoclasts and an apparent decrease in their resorptive activity.
In healthy adults, who have a relatively low rate of bone resorption, the administration of exogenous calcitonin-salmon results in decreases in serum calcium within the limits of the normal range. In healthy children and in patients whose bone resorption is more rapid, decreases in serum calcium are more pronounced in response to calcitonin-salmon.
Kidney
Studies with injectable calcitonin-salmon show increases in the excretion of filtered phosphate, calcium, and sodium by decreasing their tubular reabsorption. Comparable studies have not been conducted with Fortical nasal spray.
Gastrointestinal Tract
Some evidence from studies with injectable preparations suggests that calcitonin-salmon may have effects on the gastrointestinal tract. Short-term administration of injectable calcitonin-salmon results in marked transient decreases in the volume and acidity of gastric juice and in the volume and the trypsin and amylase content of pancreatic juice. Whether these effects continue to be elicited after each injection of calcitonin-salmon during chronic therapy has not been investigated. These studies have not been conducted with Fortical nasal spray.
Calcium Homeostasis
In two clinical studies designed to evaluate the pharmacodynamic response to calcitonin-salmon nasal spray, administration of 100-1600 International Units to healthy volunteers resulted in rapid and sustained small decreases within the normal range for both total serum calcium and serum ionized calcium. Single doses of calcitonin-salmon greater than 400 International Units did not produce any further biological response to the drug.
12.3 Pharmacokinetics
The pharmacokinetic properties of Fortical nasal spray after multiple dose administration were shown to be similar to that of a commercially available calcitonin-salmon product in healthy volunteers. Fortical nasal spray is absorbed rapidly by the nasal mucosa. In healthy volunteers approximately 3% (range 0.3%-30.6%) of a nasally administered dose is bioavailable compared to the same dose administered by intramuscular injection. Peak plasma concentrations of drug appear approximately 10 minutes after nasal administration. The terminal half-life (t1/2) of calcitonin-salmon is calculated to be about 23 minutes. There is no accumulation of the drug on repeated nasal administration at 10 hour intervals for up to 15 days. Absorption of Fortical nasal spray has not been studied in postmenopausal women.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity
The incidence of pituitary adenomas was increased in rats after one and two years of subcutaneous exposure to synthetic calcitonin-salmon. The significance of this finding to humans is unknown because pituitary adenomas are very common in rats as they age, the pituitary adenomas did not transform into metastatic tumors, there were no other clear treatment-related neoplasms, and synthetic calcitonin-salmon related neoplasms were not observed in mice after two years of dosing.
Rat findings:
The only clear neoplastic finding in rats dosed subcutaneously with synthetic calcitonin-salmon was an increase in the incidence of pituitary adenomas in male Fisher 344 rats and female Sprague Dawley rats after one year of dosing and male Sprague Dawley rats dosed for one and two years. In female Sprague Dawley rats, the incidence of pituitary adenomas after two years was high in all treatment groups (between 80% and 92% including the control groups) such that a treatment-related effect could not be distinguished from natural background incidence. The lowest dose in male Sprague Dawley rats that developed an increased incidence of pituitary adenomas after two years of dosing (1.7 International Units/kg/day) is approximately 2 times the maximum recommended intranasal dose in humans (200 International Units/day) based on body surface area conversion between rats and humans and a 20-fold conversion factor to account for decreased clinical exposure via the intranasal route. The findings suggest that calcitonin-salmon reduced the latency period for development of non-functioning pituitary adenomas.
Mouse findings:
No carcinogenicity potential was evident in male or female mice dosed subcutaneously for two years with synthetic calcitonin-salmon at doses up to 800 International Units/kg/day. The 800 International Units/kg/day dose is approximately 390 times the maximum recommended intranasal dose in humans (200 International Units) based on scaling for body surface area and a 20-fold conversion factor to account for low clinical exposure via the intranasal route.
Mutagenesis
Synthetic calcitonin-salmon tested negative for mutagenicity using Salmonella typhimurium (5 strains) and Escherichia coli (2 strains), with and without rat liver metabolic activation, and was not clastogenic in a chromosome aberration test in Chinese Hamster V79 cells. There was no evidence that calcitonin-salmon was clastogenic in the in vivo mouse micronucleus test.
14 CLINICAL STUDIES
Two randomized, placebo-controlled, two-year trials were conducted in 266 postmenopausal women who were greater than 5 years postmenopause with spinal, forearm or femoral bone mineral density (BMD) at least one standard deviation below the normal value for healthy premenopausal women (T-score < -1). In both studies, a total of 144 patients received calcitonin-salmon nasal spray 200 International Units or placebo daily. The intent-to-treat population comprised 139 patients who had at least one follow-up BMD measurement. In study 1, patients also received 500 mg daily calcium supplements, while in study 2, patients received no calcium supplementation. The primary endpoint for both studies was percent change in lumbar spine BMD at 2 years. Calcitonin-salmon nasal spray increased lumbar vertebral BMD relative to placebo in women with low bone mass who were greater than 5 years post menopause (see Table 3 below).
| Lumbar Spine Bone Mineral Density, Mean Change From Baseline (in %) at Month 24 | ||
|---|---|---|
| Study 1 (with calcium supplement) | Study 2 (no calcium supplement) |
|
| n (ITT) = 100 | n (ITT) = 39 | |
| ITT: Intent To Treat | ||
| IU: International Units | ||
| NS: nasal spray | ||
|
|
||
| Calcitonin-salmon 200 IU NS daily | +1.56 | +1.02 |
| Placebo | +0.20 | -1.85 |
| Treatment Difference | +1.36 | +2.87 |
| p-value* | < 0.05 | < 0.005 |
No effects of calcitonin-salmon nasal spray on cortical bone of the forearm or hip were demonstrated.
In clinical studies of postmenopausal osteoporosis, bone biopsy and radial bone mass assessments at baseline and after 26 months of daily injectable calcitonin-salmon indicate that calcitonin therapy results in the formation of normal bone.
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
Fortical® (calcitonin-salmon [rDNA origin]) nasal spray is presented as a metered dose solution in a 3.7 mL fill amber glass bottle with screw-on pump that contains 2200 International Units of calcitonin-salmon per mL. Following priming, the pump will deliver 200 International Units of calcitonin-salmon per activation (0.09 mL per spray). Fortical® nasal spray is provided in individual boxes containing one glass bottle with screw cap and one screw-on pump (NDC# 0245-0008-35).
Storage and Handling
Store unopened bottle in refrigerator between 2° to 8°C (36° to 46°F). Protect from freezing. After opening, store bottle in use in an upright position at 20° to 25°C (68° to 77°F). Excursions permitted to 15° to 30°C (59° to 86°F). Discard the bottle after 30 doses have been used.
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Patient Information and Instructions for Use).
- Instruct patients on pump assembly, priming of the pump, and nasal introduction of Fortical nasal spray. Although instructions for patients are supplied with the individual bottle, procedures for use should be demonstrated to each patient [see Dosage and Administration (2.2)]. Patients should notify their healthcare provider if they develop significant nasal irritation [see Warnings and Precautions (5.3)].
- Inform patients of the potential increase in risk of malignancy [see Warnings and Precautions (5.4)].
- Advise patients to maintain an adequate calcium (at least 1000 mg elemental calcium per day) and vitamin D (at least 400 International Units per day) intake [see Dosage and Administration (2.3)].
- Instruct patients to seek emergency medical help or go to the nearest hospital emergency room right away if they develop any signs or symptoms of a serious allergic reaction.
- Advise patients how to correctly store unopened and opened product [see How Supplied/Storage and Handling (16)]. Advise patients that the bottle should be discarded after 30 doses, because after 30 doses, each spray may not deliver the correct amount of medication even if the bottle is not completely empty.
Distributed by
UPSHER-SMITH LABORATORIES, INC.
Maple Grove, MN USA 55369
US Patent RE 40,182
US Patent RE 43,580
US Patent 6,440,392
Revised 0714
PATIENT INFORMATION LEAFLET
Fortical®
calcitonin-salmon (rDNA origin)
Nasal Spray
Patient Information and Instructions for Use
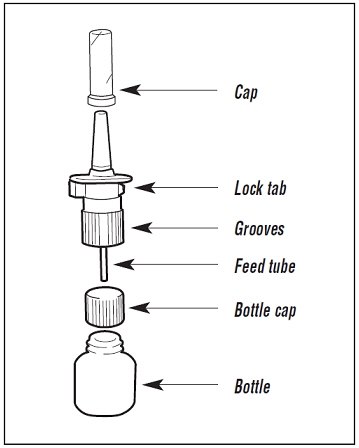
Patient Information
Fortical [fōr-tĭ-kăl]
(calcitonin-salmon [rDNA origin])
Nasal Spray
Read this Patient Information before you start using Fortical Nasal Spray and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
What is Fortical Nasal Spray?
Fortical Nasal Spray is a prescription medicine used to treat osteoporosis in women more than 5 years after menopause. Fortical Nasal Spray should be used for women who cannot use other treatments or who choose not to use other treatments for osteoporosis.
It is not known if Fortical Nasal Spray lowers the chance of having bone fractures.
Calcitonin-salmon the active ingredient in Fortical Nasal Spray has not been shown to be effective in women less than 5 years after menopause.
It is not known if Fortical Nasal Spray is safe and effective in children under 18 years of age.
Who should not use Fortical Nasal Spray?
Do not use Fortical Nasal Spray if you:
- are allergic to calcitonin-salmon or any of the ingredients in Fortical Nasal Spray. See the end of this leaflet for a complete list of ingredients in Fortical Nasal Spray.
What should I tell my healthcare provider before using Fortical Nasal Spray?
Before you use Fortical Nasal Spray, tell your healthcare provider if you:
- have any other medical conditions
- have low calcium levels in your blood
- are pregnant or plan to become pregnant. It is not known if Fortical Nasal Spray can harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if Fortical Nasal Spray passes into your breast milk. You and your healthcare provider should decide if you will use Fortical Nasal Spray or breastfeed.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements.
Especially tell your healthcare provider if you take:
- lithium. Your healthcare provider may need to change your dose of lithium while you use Fortical Nasal Spray.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I use Fortical Nasal Spray?
- For detailed instructions, see the Instructions for Use at the end of this Patient Information leaflet.
- Use Fortical Nasal Spray exactly as your healthcare provider tells you to use it.
- Do not use Fortical Nasal Spray until your healthcare provider shows you and you understand how to use it correctly.
- Use 1 spray of Fortical Nasal Spray, 1 time each day, in 1 nostril (inside your nose).
- Start with 1 spray in your left nostril on your first day, followed by 1 spray in your right nostril on the second day.
- Continue to switch nostrils for your dose each day.
- Your healthcare provider should check your nose before you start using Fortical Nasal Spray and often while you are using it.
- Tell your healthcare provider if you start to have discomfort (irritation) in your nose that bothers you while you use Fortical Nasal Spray.
- Your healthcare provider should prescribe calcium and vitamin D to help prevent low calcium levels in your blood while you use Fortical Nasal Spray.
- Take your calcium and vitamin D as your healthcare provider tells you to.
- There are 30 doses (sprays) of Fortical Nasal Spray in each bottle. After 30 doses, each spray may not give you the right amount of medicine, even if the bottle is not completely empty. Keep track of the number of doses of medicine used from your bottle.
- If you use too much Fortical Nasal Spray, call your healthcare provider or go to the nearest hospital emergency room right away.
What are the possible side effects of Fortical Nasal Spray?
Fortical Nasal Spray may cause serious side effects, including:
-
allergic reactions. Some people have had an allergic reaction when using Fortical Nasal Spray. Some reactions may be serious and can be life threatening. Call your healthcare provider or go to the nearest hospital emergency room right away if you have any of these symptoms of an allergic reaction.
- trouble breathing
- swelling of your face, throat or tongue
- fast heartbeat
- chest pain
- feel dizzy or faint
If you might be allergic to calcitonin-salmon, your healthcare provider should do a skin test before you use Fortical Nasal Spray.
-
low calcium levels in your blood (hypocalcemia). Fortical Nasal Spray may lower the calcium in your blood. If you have low blood calcium before you start using Fortical Nasal Spray, it may get worse during treatment. Your low blood calcium must be treated before you use Fortical Nasal Spray. Most people with low blood calcium levels do not have symptoms, but some people may have symptoms. Call your healthcare provider right away if you have any of these symptoms of low blood calcium:
- numbness or tingling in your fingers, toes, or around your mouth
- do blood tests while you use Fortical Nasal Spray
- prescribe calcium and vitamin D to help prevent low calcium levels in your blood while you use Fortical Nasal Spray.
Take your calcium and vitamin D as your healthcare provider tells you to.
- nose irritation
Irritation of your nose can happen while you are using Fortical Nasal Spray, especially if you are over 65 years of age. Call your healthcare provider right away if you have any of these symptoms of nose irritation:
-
- crusting
- dryness
- redness or swelling
- nose sores (ulcers)
- nose bleeds
Your healthcare provider may stop your treatment with Fortical Nasal Spray until your nose irritation symptoms go away.
- risk of cancer
People who use calcitonin-salmon, the medicine in Fortical Nasal Spray, may have an increased risk of cancer. Your healthcare provider should check while you are using Fortical to see if it is still right for you.
- increase of certain cells (sediment) in your urine
Your healthcare provider should test your urine often while you are using Fortical Nasal Spray.
The most common side effects of Fortical® Nasal Spray include:
- runny nose
- nose bleeds
- nasal problems
- back pain
- muscle pain
- headache
These are not all the possible side effects of Fortical Nasal Spray. For more information, ask your healthcare provider or pharmacist.
Tell your healthcare provider right away if you have any side effect that bothers you or does not go away.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How do I store Fortical Nasal Spray?
- Store open bottles of Fortical Nasal Spray at room temperature , 68°F to 77°F (20°C to 25°C).
- Store unopened bottles of Fortical Nasal Spray in the refrigerator between 36°F to 46°F (2°C to 8°C). Do not freeze.
- Store Fortical Nasal Spray bottles in an upright position.
- Safely throw away Fortical Nasal Spray in the trash after you have used 30 doses (sprays).
Keep Fortical Nasal Spray and all other medicines out of the reach of children.
General Information about the safe and effective use of Fortical Nasal Spray.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Fortical Nasal Spray for a condition for which it was not prescribed. Do not give Fortical Nasal Spray to other people, even if they have the same symptoms you have. It may harm them.
This Patient Information Leaflet summarizes the most important information about Fortical Nasal Spray. If you would like more information about Fortical Nasal Spray talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about Fortical Nasal Spray that is written for health professionals.
For more information, go to www.upsher-smith.com or call 1-888-650-3789.
What are the ingredients in Fortical Nasal Spray?
Active Ingredient: calcitonin-salmon
Inactive Ingredients: sodium chloride, citric acid, phenylethyl alcohol, benzyl alcohol, polysorbate 80, hydrochloric acid or sodium hydroxide and purified water.
Instructions for Use
Fortical [fōr-tĭ-kăl]
(calcitonin-salmon [rDNA origin])
Nasal Spray
For Nasal Use Only
Important information about your Fortical Nasal Spray:
- A single spray of Fortical Nasal Spray contains 1 daily dose of medicine.
- Each Fortical Nasal Spray bottle contains the right amount of medicine. The bottle may not be completely filled to the top. This is normal.
- This package contains 1 bottle of Fortical Nasal Spray and 1 screw-on pump.
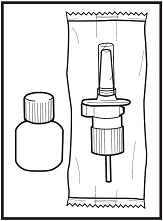
- Store unopened bottles of Fortical Nasal Spray in the refrigerator between 36°F to 46°F (2°C to 8°C). Do not freeze.
- After you open your bottle of Fortical Nasal Spray, store it at room temperature between 68°F to 77°F (20°C to 25°C) in an upright position. Do not shake the bottle.
Preparing your Fortical Nasal Spray
Remove the bottle from your refrigerator and let it reach room temperature.
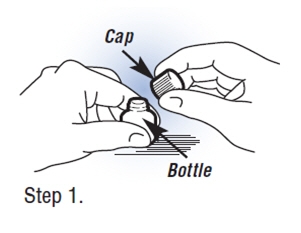 | Step 1.Opening the Bottle. Set bottle on a flat surface like a table or counter top. Hold the bottle firmly while unscrewing the cap to avoid spilling any Fortical Nasal Spray. Important: If your pharmacist attached the pump for you, skip to Step 4. |
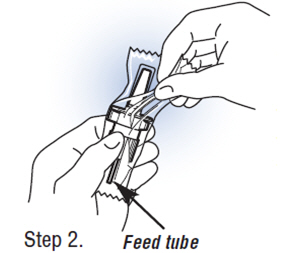 | Step 2.Unwrapping the Pump. Carefully cut the wrapper with scissors or knife, and tear away from the pump. Be careful not to cut, bend, or touch the feed tube at the bottom. |
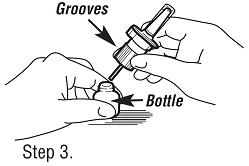 | Step 3. Attaching the Pump. Hold the bottle on a flat surface (like a table), and screw the pump onto bottle by gripping the grooves of the pump and twisting clockwise until the pump is tightly sealed to the bottle. |
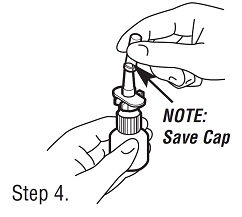 | Step 4. Removing Cap. To remove the cap from the pump, twist slightly while pulling up. Save the cap to replace after each use. |
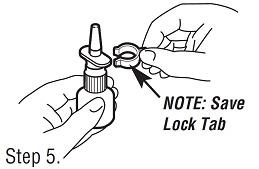 Save the lock tab, and push it back onto the pump after each use to avoid accidentally wasting any Fortical Nasal Spray Save the lock tab, and push it back onto the pump after each use to avoid accidentally wasting any Fortical Nasal Spray | Step 5. Removing the Lock Tab. Grasp the lock tab between the thumb and forefinger, while holding the bottle on a flat surface. Pull sideways to remove the tab and unlock the pump. |
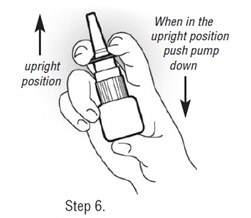 | Step 6. Prime New Pump Before First Use.
Before you use a new bottle of Fortical Nasal Spray for the first time, you will need to prime it. This will make sure you get the right dose of medicine each time you use it. After you attach the pump to the bottle, and the pump and bottle have reached room temperature, you should:
|
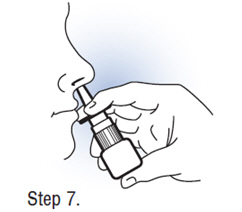 | Step 7. Using Fortical Nasal Spray
|
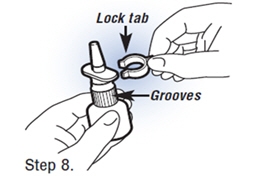 | Step 8. Replace The Lock Tab After Use Then Clean the Spray Tip.
|
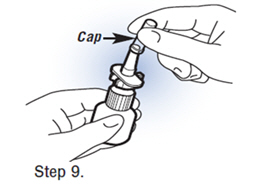 | Step 9. Replace The Cap.
|
| Step 10. Storing your Fortical Nasal Spray. Store Fortical Nasal Spray in an upright position. Tighten the pump securely to the bottle (see Step 3 and 4 in the Patient Instructions for Use). This will help to make sure there is a good seal between the pump and the bottle. | |
When should I throw away Fortical Nasal Spray?
- Unopened, refrigerated bottles can be used until the expiration date stamped on the bottle and box.
- Throw away Fortical Nasal Spray after you use 30 doses (sprays).
This Patient Information and Instructions for Use has been approved by the U.S. Food and Drug Administration.
Distributed by
UPSHER-SMITH LABORATORIES, INC.
Maple Grove, MN USA 55369
www.upsher-smith.com
Revised 0714
PRINCIPAL DISPLAY PANEL - 3.7 mL Bottle Carton
NDC: 0245-0008-35
FORTICAL®
(calcitonin-salmon
[rDNA origin])
Nasal Spray
200 International Units/dose
2200 IU/mL
REFRIGERATE
UNTIL OPENED
at 2° to 8°C (36° to 46°F)
FOR INTRANASAL USE ONLY
30 doses
Each carton contains:
1 x 3.7 mL fill bottle and
1 spray applicator
Rx only
UPSHER-SMITH

| FORTICAL
calcitonin salmon spray, metered |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Upsher-Smith Laboratories, Inc. (047251004) |