NEUAC- clindamycin phosphate and benzoyl peroxide gel
Neuac by
Drug Labeling and Warnings
Neuac by is a Prescription medication manufactured, distributed, or labeled by Medimetriks Pharmaceuticals, Padagis Israel. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use Neuac® (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5% safely and effectively. See full prescribing information for Neuac® (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5%.
Neuac® (CLINDAMYCIN PHOSPHATE and BENZOYL PEROXIDE) gel, for topical use.
Initial U.S. Approval: 2000INDICATIONS AND USAGE
Neuac® (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5% is a combination of clindamycin phosphate (a lincosamide antibacterial) and benzoyl peroxide indicated for the topical treatment of inflammatory acne vulgaris. (1.1)
Limitation of Use:
Neuac® (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5% has not been demonstrated to have any additional benefit when compared with benzoyl peroxide alone in the same vehicle when used for the treatment of non-inflammatory acne. (1.2)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Gel, 1.2%/5%: Each gram of Neuac® (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5% contains 12 mg clindamycin phosphate (equivalent to 10 mg of clindamycin) and 50 mg benzoyl peroxide. (3)
CONTRAINDICATIONS
Neuac® (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5% is contraindicated in:
WARNINGS AND PRECAUTIONS
- Colitis: Clindamycin can cause severe colitis, which may result in death. Diarrhea, bloody diarrhea, and colitis (including pseudomembranous colitis) have been reported with the use of clindamycin. Neuac® (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5% should be discontinued if significant diarrhea occurs. (5.1)
- Ultraviolet light and environmental exposure (including use of tanning beds or sun lamps): Minimize sun exposure following drug application. (5.2)
ADVERSE REACTIONS
- The most common local adverse reactions (≥5%) are erythema, peeling, dryness, and burning. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Medimetriks Pharmaceuticals, Inc. at 973-882-7512 or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Neuac® (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5% should not be used in combination with erythromycin-containing products because of its clindamycin component. (7.1)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 11/2015
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Indication
1.2 Limitations of Use
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Hypersensitivity
4.2 Colitis/Enteritis
5 WARNINGS AND PRECAUTIONS
5.1 Colitis
5.2 Ultraviolet Light and Environmental Exposure
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Erythromycin
7.2 Concomitant Topical Medications
7.3 Neuromuscular Blocking Agents
7.4 Topical Sulfone Products
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
16.3 Dispensing Instructions for the Pharmacist
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
Apply a thin layer of Neuac® (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5% to the face once daily, in the evening or as directed by the physician. The skin should be gently washed, rinsed with warm water, and patted dry before applying Neuac® (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5%. Avoid the eyes, mouth, lips, mucous membranes, or areas of broken skin.
Neuac® (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5% is not for oral, ophthalmic, or intravaginal use.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
4.1 Hypersensitivity
Neuac® (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5% is contraindicated in those individuals who have shown hypersensitivity to clindamycin, benzoyl peroxide, any components of the formulation, or lincomycin. Anaphylaxis, as well as allergic reactions leading to hospitalization, has been reported in postmarketing use with clindamycin phosphate and benzoyl peroxide gel, 1.2%/5% [see Adverse Reactions (6.2)].
4.2 Colitis/Enteritis
Neuac® (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5% is contraindicated in those individuals with a history of regional enteritis, ulcerative colitis, pseudomembranous colitis, or antibiotic-associated colitis [see Warnings and Precautions (5.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Colitis
Systemic absorption of clindamycin has been demonstrated following topical use of clindamycin. Diarrhea, bloody diarrhea, and colitis (including pseudomembranous colitis) have been reported with the use of topical and systemic clindamycin. If significant diarrhea occurs, Neuac® (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5% should be discontinued.
Severe colitis has occurred following oral and parenteral administration of clindamycin with an onset of up to several weeks following cessation of therapy. Antiperistaltic agents such as opiates and diphenoxylate with atropine may prolong and/or worsen severe colitis. Severe colitis may result in death.
Studies indicate a toxin(s) produced by Clostridia is one primary cause of antibiotic-associated colitis. The colitis is usually characterized by severe persistent diarrhea and severe abdominal cramps and may be associated with the passage of blood and mucus. Stool cultures for Clostridium difficile and stool assay for C. difficile toxin may be helpful diagnostically.
5.2 Ultraviolet Light and Environmental Exposure
Benzoyl peroxide, a component of Neuac® (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5%, may cause increased sensitivity to sunlight. Minimize sun exposure (including use of tanning beds or sun lamps) following drug application. [See Nonclinical Toxicology (13.1)] Patients who may be required to have considerable sun exposure due to occupation and those with inherent sensitivity to the sun should exercise particular caution.
-
6 ADVERSE REACTIONS
The following adverse reaction is described in more detail in the Warnings and Precautions section of the label:
- Colitis [see Warnings and Precautions (5.1)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
During clinical trials, 397 subjects used clindamycin phosphate and benzoyl peroxide gel, 1.2%/5% once daily for 11 weeks for the treatment of moderate to moderately severe facial acne vulgaris. All subjects were graded for facial local skin reactions (erythema, peeling, burning, and dryness) on the following scale: 0 = absent, 1 = mild, 2 = moderate, and 3 = severe. The percentage of subjects that had symptoms present before treatment (at baseline) and during treatment is presented in Table 1.
Table 1. Local Skin Reactions With Use Of Clindamycin Phosphate and Benzoyl Peroxide Gel, 1.2%/5% Combined Results From Five Trials (n=397) % of Subjects Using Clindamycin Phosphate and Benzoyl Peroxide Gel, 1.2%/5% with Symptom Present Before Treatment (Baseline) During Treatment Symptom Mild Moderate Severe Mild Moderate Severe Erythema 28% 3% 0 26% 5% 0 Peeling 6% <1% 0 17% 2% 0 Burning 3% <1% 0 5% <1% 0 Dryness 6% <1% 0 15% 1% 0 (Percentages derived by number of subjects receiving clindamycin phosphate and benzoyl peroxide gel, 1.2%/5% with symptom score/number of enrolled subjects receiving clindamycin phosphate and benzoyl peroxide gel, 1.2%/5%).
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of clindamycin phosphate and benzoyl peroxide gel, 1.2%/5%. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Anaphylaxis, as well as allergic reactions leading to hospitalization, has been reported in postmarketing use with clindamycin phosphate and benzoyl peroxide gel, 1.2%/5%.
Urticaria, application site reactions, including discoloration have been reported.
-
7 DRUG INTERACTIONS
7.1 Erythromycin
Avoid using Neuac® (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5% in combination with erythromycin-containing products due to its clindamycin component. In vitro studies have shown antagonism between erythromycin and clindamycin. The clinical significance of this in vitro antagonism is not known.
7.2 Concomitant Topical Medications
Concomitant topical acne therapies should be used with caution since a possible cumulative irritancy effect may occur, especially with the use of peeling, desquamating, or abrasive agents. If irritancy or dermatitis occurs, reduce frequency of application or temporarily interrupt treatment and resume once the irritation subsides. Treatment should be discontinued if the irritation persists.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C.
There are no adequate and well-controlled studies in pregnant women treated with Clindamycin Phosphate and Benzoyl Peroxide Gel, 1.2%/5%. Neuac® (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5% should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Developmental toxicity studies performed in rats and mice using oral doses of clindamycin up to 600 mg per kg per day (240 and 120 times the amount of clindamycin in the highest recommended adult human dose based on mg per m2, respectively) or subcutaneous doses of clindamycin up to 250 mg per kg per day (100 and 50 times the amount of clindamycin in the highest recommended adult human dose based on mg per m2, respectively) revealed no evidence of teratogenicity.
8.3 Nursing Mothers
It is not known whether Neuac® (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5% is excreted into human milk after topical application.
However, orally and parenterally administered clindamycin has been reported to appear in breast milk. Because of the potential for serious adverse reactions in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. Because many drugs are excreted in human milk, caution should be exercised when Neuac® (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5% is administered to a nursing woman.
-
11 DESCRIPTION
Neuac® (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5% is a fixed combination product with two active ingredients in a white, opaque, aqueous gel formulation.
Clindamycin phosphate is a water soluble ester of the semi-synthetic antibiotic produced by a 7(S)-chloro-substitution of the 7(R)-hydroxyl group of the parent antibiotic lincomycin.
Clindamycin phosphate is C18H34ClN2O8PS. The structural formula for clindamycin phosphate is represented below:
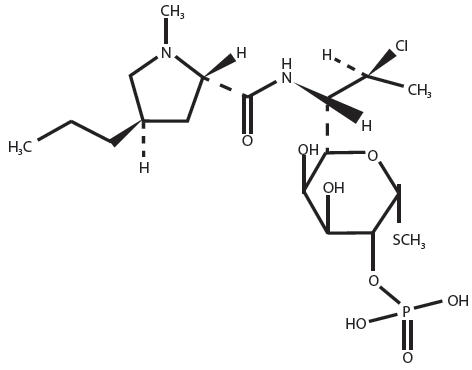
Clindamycin phosphate has a molecular weight of 504.97 and its chemical name is methyl 7-chloro-6,7,8-trideoxy-6-(1-methyl-trans-4-propyl-L-2-pyrrolidinecarboxamido)-1-thio-L-threo-α-D-galacto-octopyranoside 2-(dihydrogen phosphate).
Benzoyl peroxide is C14H10O4. It has the following structural formula:
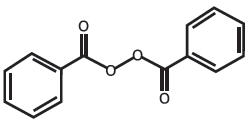
Benzoyl peroxide has a molecular weight of 242.23.
Each gram of Neuac® (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5% contains 10 mg (1%) clindamycin, as clindamycin phosphate, and 50 mg (5%) benzoyl peroxide in a base consisting of carbomer homopolymer (type B), hydrochloric acid, methylparaben, dimethicone, propylparaben, purified water and sodium hydroxide.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Clindamycin: Clindamycin is a lincosamide antibacterial [see Clinical Pharmacology (12.4)].
12.3 Pharmacokinetics
A comparative trial of the pharmacokinetics of clindamycin phosphate and benzoyl peroxide gel, 1.2%/5% and 1% clindamycin solution alone in 78 subjects indicated that mean plasma clindamycin levels during the 4-week dosing period were less than 0.5 ng/mL for both treatment groups.
Benzoyl peroxide has been shown to be absorbed by the skin where it is converted to benzoic acid. Less than 2% of the dose enters systemic circulation as benzoic acid.
12.4 Microbiology
Clindamycin binds to the 50S ribosomal subunits of susceptible bacteria and prevents elongation of peptide chains by interfering with peptidyl transfer, thereby suppressing protein synthesis.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Benzoyl peroxide has been shown to be a tumor promoter and progression agent in a number of animal studies. Benzoyl peroxide in acetone at doses of 5 and 10 mg administered twice per week induced squamous cell skin tumors in transgenic TgAC mice in a study using 20 weeks of topical treatment. The clinical significance of this is unknown.
In a 2-year dermal carcinogenicity study in mice, treatment with clindamycin phosphate and benzoyl peroxide gel, 1.2%/5% at doses up to 8,000 mg per kg per day (16 times the highest recommended adult human dose of 2.5 g clindamycin phosphate and benzoyl peroxide gel, 1.2%/5%, based on mg per m2) did not cause an increase in skin tumors. However, topical treatment with another formulation containing 1% clindamycin and 5% benzoyl peroxide at doses of 100, 500, or 2,000 mg per kg per day caused a dose-dependent increase in the incidence of keratoacanthoma at the treated skin site of male rats in a 2-year dermal carcinogenicity study in rats.
In a 52-week photocarcinogenicity study in hairless mice (40 weeks of treatment followed by 12 weeks of observation), the median time to onset of skin tumor formation decreased and the number of tumors per mouse increased relative to controls following chronic concurrent topical treatment with clindamycin phosphate and benzoyl peroxide gel, 1.2%/5% and exposure to ultraviolet radiation.
Genotoxicity studies were not conducted with clindamycin phosphate and benzoyl peroxide gel, 1.2%/5%. Clindamycin phosphate was not genotoxic in Salmonella typhimurium or in a rat micronucleus test. Benzoyl peroxide has been found to cause DNA strand breaks in a variety of mammalian cell types, to be mutagenic in Salmonella typhimurium tests by some but not all investigators, and to cause sister chromatid exchanges in Chinese hamster ovary cells.
Studies have not been performed with clindamycin phosphate and benzoyl peroxide gel, 1.2%/5% or benzoyl peroxide to evaluate the effect on fertility. Fertility studies in rats treated orally with up to 300 mg per kg per day of clindamycin (approximately 120 times the amount of clindamycin in the highest recommended adult human dose of 2.5 g clindamycin phosphate and benzoyl peroxide gel, 1.2%/5%, based on mg per m2) revealed no effects on fertility or mating ability.
-
14 CLINICAL STUDIES
In five randomized, double-blind clinical trials of 1,319 subjects, 397 used clindamycin phosphate and benzoyl peroxide gel, 1.2%/5%, 396 used benzoyl peroxide, 349 used clindamycin, and 177 used vehicle. Subjects were instructed to wash the face, wait 10 to 20 minutes, and then apply medication to the entire face, once daily in the evening before retiring. Clindamycin phosphate and benzoyl peroxide gel, 1.2%/5% applied once daily for 11 weeks was significantly more effective than vehicle, benzoyl peroxide, and clindamycin in the treatment of inflammatory lesions of moderate to moderately severe facial acne vulgaris in three of the five trials (Trials 1, 2, and 5). Subjects were evaluated and acne lesions counted at each clinical visit at Weeks 2, 5, 8, 11.
The primary efficacy measures were the lesion counts and the investigator's global assessment evaluated at Week 11. Percent reductions in inflammatory lesion counts after treatment for 11 weeks in these 5 trials are shown in Table 2.
Table 2. Mean Percent Reduction In Inflammatory Lesion Counts Treatment Trial 1
(n = 120)Trial 2
(n = 273)Trial 3
(n = 280)Trial 4
(n = 288)Trial 5
(n = 358)Clindamycin Phosphate and Benzoyl Peroxide Gel, 1.2%/5% 65% 56% 42% 57% 52% Benzoyl Peroxide 36% 37% 32% 57% 41% Clindamycin 34% 30% 38% 49% 33% Vehicle 19% -0.4% 29% -- 29% The group treated with clindamycin phosphate and benzoyl peroxide gel, 1.2%/5% showed greater overall improvement in the investigator's global assessment than the benzoyl peroxide, clindamycin, and vehicle groups in three of the five trials (Trials 1, 2, and 5).
Clinical trials have not adequately demonstrated the effectiveness of clindamycin phosphate and benzoyl peroxide gel, 1.2%/5% versus benzoyl peroxide alone in the treatment of non-inflammatory lesions of acne.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Neuac® (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5% is a white, opaque gel. It is supplied as follows:
- 45 gram tube NDC 43538-176-45
-
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Patient Information).
- Patients who develop allergic reactions such as severe swelling or shortness of breath should discontinue use and contact their physician immediately.
- Neuac® (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5% may cause irritation such as erythema, scaling, itching, or burning, especially when used in combination with other topical acne therapies.
- Excessive or prolonged exposure to sunlight should be limited. To minimize exposure to sunlight, a hat or other clothing should be worn. Sunscreen may also be used.
- Neuac® (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5% may bleach hair or colored fabric.
- Neuac® (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5% may cause skin and facial hair to temporarily change color (yellow/orange) when used with topical sulfone products.
- SPL UNCLASSIFIED SECTION
-
PATIENT INFORMATION
Neuac® (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5%
Important: For use on the skin only (topical use). Do not get Neuac® (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5% in your mouth, eyes, vagina, or on your lips. Read this Patient Information before you start using Neuac® (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5% and each time you get a refill. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or your treatment.
What is Neuac® (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5%?
Neuac® Gel is a prescription medicine used on the skin (topical) to treat inflamed acne in people 12 years and older.
Who should not use Neuac® (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5%?
Do not use Neuac® Gel if you have:
- had an allergic reaction to clindamycin, lincomycin, benzoyl peroxide, or any of the ingredients in Neuac® (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5%. See the end of this leaflet for a complete list of ingredients in Neuac® Gel.
- Crohn's disease or ulcerative colitis.
- had inflammation of the colon (colitis) with past antibiotic use.
What should I tell my healthcare provider before using Neuac® (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5%?
Before using Neuac® Gel, tell your healthcare provider about all of your medical conditions, including if you:
- plan to have surgery with general anesthesia.
- are sensitive to sunlight.
- are pregnant or plan to become pregnant. It is not known if Neuac® Gel, will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if Neuac® Gel passes into your breast milk. One of the medicines in Neuac® Gel is clindamycin. Clindamycin when taken by mouth or by injection has been reported to appear in breast milk. You and your healthcare provider should decide if you will use Neuac® Gel while breastfeeding.
Tell your healthcare provider about all the medicines you take, including prescription or over-the-counter medicines, vitamins, herbal supplements, and skin products you use. Using other topical acne products may increase the irritation of your skin when used with Neuac® (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5%.
- Especially tell your healthcare provider if you take a medicine that contains erythromycin. Neuac® Gel should not be used with products that contain erythromycin.
- Neuac® Gel may cause skin and facial hair to temporarily change color (yellow or orange) when used with topical products that contain sulfones.
How should I use Neuac® (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5%?
- Use Neuac® Gel exactly as your healthcare provider tells you to use it.
- Before you apply Neuac® Gel, wash your face gently with a mild soap, rinse with warm water, and pat the skin dry.
- Apply a thin layer of Neuac® Gel to your face 1 time a day, in the evening or as directed by your healthcare provider. Wash your hands with soap and water after applying Neuac® Gel.
- Do not get Neuac® Gel in your mouth, eyes, nose, vagina, or on your lips. Do not get Neuac® Gel on cuts or open wounds.
What should I avoid while using Neuac® (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5%?
- Limit your time in sunlight. Avoid using tanning beds or sun lamps. If you have to be in sunlight, wear a wide-brimmed hat or other protective clothing. Sunscreen may also be used.
- Talk to your healthcare provider if you spend a lot of time in the sun.
- Neuac® Gel may bleach hair or colored fabric.
What are the possible side effects with Neuac® (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5%?
Neuac® Gel may cause serious side effects, including:
- Inflammation of the colon (colitis). Stop using Neuac® Gel and call your healthcare provider right away if you have severe watery diarrhea, or bloody diarrhea.
-
Allergic reactions. Stop using Neuac® Gel and call your healthcare provider or get help right away if you have any of the following symptoms:
- severe itching
- swelling of your face, eyes, lips, tongue, or throat
- trouble breathing
The most common side effects with Neuac® (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5% are skin reactions and may include redness, peeling, dryness, and burning.
These are not all the possible side effects with Neuac® Gel.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store Neuac® (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5%?
- Store Neuac® Gel at room temperature up to 25°C (77°F). Do not freeze Neuac® Gel.
- The expiration date of Neuac® Gel is 60 days from the date when you fill your prescription.
- Safely throw away expired Neuac® Gel.
- Keep the tube tightly closed.
Keep Neuac® Gel and all medicines out of the reach of children.
General information about Neuac® (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5%
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Neuac® Gel for a condition for which it was not prescribed. Do not give Neuac® Gel to other people, even if they have the same symptoms you have. It may harm them.
If you would like more information, talk with your healthcare provider. You can also ask your pharmacist or healthcare provider for information about Neuac® Gel that is written for health professionals.
What are the ingredients in Neuac® (clindamycin phosphate and benzoyl peroxide) Gel, 1.2%/5%?
Active Ingredients: clindamycin phosphate 1.2% and benzoyl peroxide 5%
Inactive ingredients: carbomer homopolymer (type B), hydrochloric acid, methylparaben, dimethicone, propylparaben, purified water and sodium hydroxide.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Manufactured For Medimetriks Pharmaceuticals, Inc.
383 Route 46 West, Fairfield, NJ 07004-2402 USAManufactured By Perrigo, Yeruham, Israel Made in Israel
Distributed By Perrigo, Allegan, MI 49010
Rev 11/2015
IP037-R1
5M400 EK J1 -
PRINCIPAL DISPLAY PANEL - 45 g Tube Carton
NDC: 43538-176-45
Rx Only
Neuac®
(clindamycin phosphate and
benzoyl peroxide) Gel, 1.2%/5%For External Use Only
45 g
MEDIMETRIKS
PHARMACEUTICALS, INC.
-
INGREDIENTS AND APPEARANCE
NEUAC
clindamycin phosphate and benzoyl peroxide gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 43538-176 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength clindamycin phosphate (UNII: EH6D7113I8) (clindamycin - UNII:3U02EL437C) clindamycin 10 mg in 1 g benzoyl peroxide (UNII: W9WZN9A0GM) (benzoyl peroxide - UNII:W9WZN9A0GM) benzoyl peroxide 50 mg in 1 g Inactive Ingredients Ingredient Name Strength hydrochloric acid (UNII: QTT17582CB) methylparaben (UNII: A2I8C7HI9T) dimethicone (UNII: 92RU3N3Y1O) propylparaben (UNII: Z8IX2SC1OH) water (UNII: 059QF0KO0R) sodium hydroxide (UNII: 55X04QC32I) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 43538-176-45 1 in 1 CARTON 06/15/2014 1 45 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090979 06/15/2014 Labeler - Medimetriks Pharmaceuticals (019903816) Establishment Name Address ID/FEI Business Operations Perrigo Israel 600093611 MANUFACTURE(43538-176)
Trademark Results [Neuac]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 NEUAC 85673919 4597726 Live/Registered |
Medimetriks Pharmaceuticals, Inc. 2012-07-11 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.