SINUVA- mometasone furoate implant
Sinuva by
Drug Labeling and Warnings
Sinuva by is a Prescription medication manufactured, distributed, or labeled by Intersect ENT, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use SINUVA™ safely and effectively. See full prescribing information for SINUVA.
SINUVA (mometasone furoate) sinus implant
Initial U.S. Approval: 1987INDICATIONS AND USAGE
SINUVA™ Sinus Implant is a corticosteroid-eluting (mometasone furoate) implant indicated for the treatment of nasal polyps in patients ≥ 18 years of age who have had ethmoid sinus surgery. (1)
DOSAGE AND ADMINISTRATION
- The SINUVA Sinus Implant is loaded into a Delivery System and placed in the ethmoid sinus under endoscopic visualization. The Implant may be left in the sinus to gradually release the corticosteroid over 90 days. The Implant can be removed at Day 90 or earlier at the physician's discretion using standard surgical instruments. (2.2)
- To be inserted by physicians trained in otolaryngology. (2.3)
- Repeat administration has not been studied. (2.4)
DOSAGE FORMS AND STRENGTHS
One SINUVA Sinus Implant system contains 1350 mcg of mometasone furoate and a sterile Delivery System. (3)
CONTRAINDICATIONS
- Patients with known hypersensitivity to mometasone furoate and any of the ingredients of the SINUVA Sinus Implant. (4)
WARNINGS AND PRECAUTIONS
- Monitor nasal mucosa adjacent to the SINUVA Sinus Implant for any signs of bleeding (epistaxis), irritation, infection, or perforation. Avoid use in patients with nasal ulcers or trauma. (5.1)
- Monitor patients with a change in vision or with a history of increased intraocular pressure, glaucoma, and/or cataracts closely. (5.2)
- Hypersensitivity reactions, including rash, pruritus, and angioedema, have been reported with use of corticosteroids. (5.3)
- Potential worsening of existing tuberculosis; fungal, bacterial, viral, or parasitic infection; or ocular herpes simplex. More serious or even fatal course of chickenpox or measles in susceptible patients. Use caution in patients with the above because of the potential for worsening of these infections. (5.4)
- If corticosteroid effects such as hypercorticism and adrenal suppression appear in patients, consider sinus implant removal. (5.5)
ADVERSE REACTIONS
The most common adverse reactions (in more than 1% of subjects) were bronchitis, nasopharyngitis, otitis media, headache, presyncope, asthma, and epistaxis. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Intersect ENT at 1-866 531-6004 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 12/2017
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosing
2.2 General Instructions
2.3 Health Care Provider Training
2.4 Repeat Administration
2.5 Placement of SINUVA Sinus Implant
2.6 Removal Instructions
3 DOSAGE FORM AND STRENGTH
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Local Effects
5.2 Ocular Effects
5.3 Hypersensitivity Reactions
5.4 Immunosuppression
5.5 Hypercorticism and Adrenal Suppression
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Post-Marketing Experience
7 DRUG INTERACTIONS
7.1 Inhibitors of Cytochrome P450 3A4
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
17.1 Risks Relating to the Insertion and Removal Procedure
17.2 Local Effects
17.3 Hypersensitivity Reactions
17.4 Immunosuppression
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.2 General Instructions
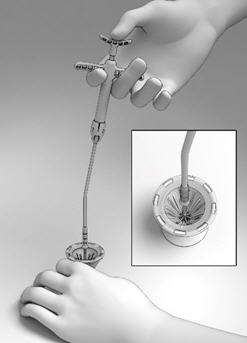
The SINUVA Sinus Implant (Figure 1) is loaded into a Delivery System and placed in the ethmoid sinus under endoscopic visualization. The SINUVA Sinus Implant is made from bioabsorbable polymers designed to gradually soften over time. The SINUVA Sinus Implant may be left in the sinus to gradually release the corticosteroid over 90 days. The SINUVA Sinus Implant can be removed at day 90 or earlier at the physician's discretion using standard surgical instruments.
2.3 Health Care Provider Training
The SINUVA Sinus Implant is to be used by physicians trained in otolaryngology. Specialized training is not required for these physicians.
2.4 Repeat Administration
There are no studies evaluating repeat implantation of the SINUVA Sinus Implant.
2.5 Placement of SINUVA Sinus Implant
The SINUVA Sinus Implant is designed for single patient use only. Do not reprocess or reuse.
- Do not use if the package is open, the package or product is damaged, or has evidence of gross contamination.
- Special care should be taken to avoid bending, twisting, or damaging the implant.
- The implant is not designed to be modified by the physician.
- The implant is not intended to be compressed and loaded into the Delivery System more than two times. The implant must be placed under endoscopic visualization.
Patient Preparation
The patient should be prepared following routine protocols for in-office sinonasal endoscopic procedures.
Implant Preparation
Remove the Crimper (Figure 2) and the Delivery System (Figure 3) from their protective packaging using sterile technique. Inspect the SINUVA Sinus Implant located inside of the Crimper (Figure 2). Do not remove the Implant from the Crimper. Prior to use, the SINUVA Sinus Implant must be crimped and loaded into the Delivery System.
If the SINUVA Sinus Implant is not fully seated inside of the Crimper, secure the SINUVA Sinus Implant before proceeding. See instructions to secure the SINUVA Sinus Implant (Figure 12–15).
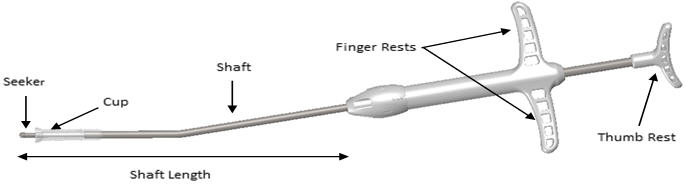
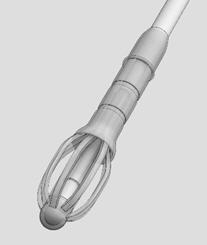
IMPLANT Length (nominal): 20 mm Expanded Diameter (nominal): 34 mm DELIVERY SYSTEM Shaft Length 117 mm Figure 1: Implant
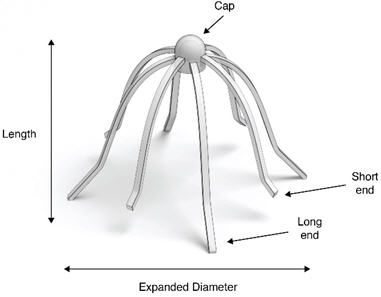
Figure 2: Crimper with Implant

Figure 3: Delivery System
- Place the Crimper on a flat surface and hold to prevent any potential slipping of the Crimper during loading of the SINUVA Sinus Implant into the Delivery System. Orient the Crimper such that the short ends of the Implant are in the 12 o'clock and 6 o'clock position (Figure 4)
Figure 4

- Grasp the Delivery System with the index and middle fingers on the left or right hand using the Finger Rests and the thumb in the Thumb Rest (Figure 5).
Figure 5
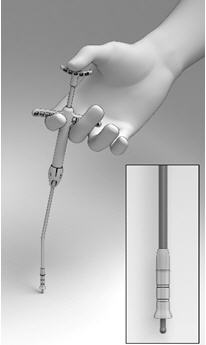
- Pull back on the Finger Rests while pressing down on the Thumb Rest to retract the Cup, and expose the Seeker (Figure 6).
Figure 6
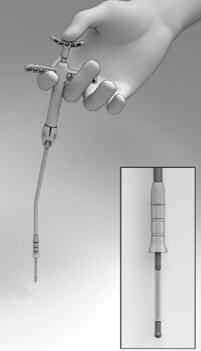
- Position the tip of the Seeker with its 10° angled tip downwards toward the user in the depression in the center of the SINUVA Sinus Implant (Figure 7). The distal end of the angled shaft must be in a vertical position, perpendicular to the Crimper, during positioning. Ensure the plane of the angled tip is in the same plane as the short ends of the Implant that were oriented in the 12 o'clock / 6 o'clock position in step 1.
Figure 7
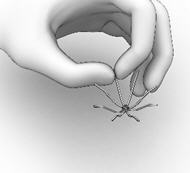
- With the Thumb Rest depressed, gradually apply perpendicular downward force to the SINUVA Sinus Implant until the ends of the Implant collapse around the Seeker of Delivery System (Figure 8). Make sure that the Finger Rests are not released while pushing downwards.
Figure 8

- The SINUVA Sinus Implant should crimp in a radial fashion onto the Seeker. Implant ends should not cross over or past each other when being crimped onto the Seeker by the Crimper.
- While maintaining steady downward pressure on the Thumb Rest, slowly release the Finger Rests with the index and middle fingers until the Cup lowers and captures all ends of the SINUVA Sinus Implant (Figure 9). If necessary, adjust the position of the Delivery System with slight circular movements, slightly lifting and then lowering the Cup into position to ensure that all eight ends of the SINUVA Sinus Implant are secured within the Cup.
Figure 9

- Apply a downward push on the Delivery System to ensure that the SINUVA Sinus Implant is secured in the Cup (Figure 10). This will also ensure the Implant is compressed to its smallest profile for insertion.
Figure 10

- Retract the Delivery System from the Crimper. The SINUVA Sinus Implant should remain symmetrically loaded in the Cup of the Delivery System (Figure 11).
Figure 11
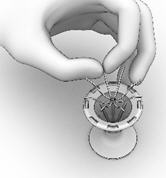
CAUTION: Do not leave the SINUVA Sinus Implant in the crimped state for more than 5 minutes prior to placement.
Instructions to Secure the SINUVA Sinus Implant in the Crimper
If necessary, the Implant may be reloaded into the Crimper for a second time.
CAUTION: The SINUVA Sinus Implant should not be used if the second attempt to crimp is unsuccessful.
- Hold the SINUVA Sinus Implant by one end as shown in Figure 12.
Figure 12
- Holding the SINUVA Sinus Implant with the dome-shaped Cap positioned downward (Figure 13), place the Implant back into the Crimper.
Figure 13
- Ensure that each Implant is secured in the Crimper by pressing down on the center of the Implant until all ends of the Implant are below the rim of the Crimper (Figure 14).
Figure 14
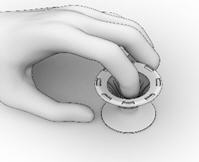
- Inspect the Implant and the Crimper to ensure that all the Implant ends are secured below the rim of the Crimper (Figure 15). Return to Implant Preparation Step 1 for instructions on how to load the re-secured implant into the delivery system.
Figure 15

Instructions for the SINUVA Sinus Implant Placement
Advance the Delivery System under endoscopic visualization into the ethmoid sinus cavity.
- Ensure that the Delivery System is oriented such that the 10° curvature of the distal tip is curved superiorly. Insert the Delivery System such that the Shaft is parallel to roof of ethmoid sinus.
- If the SINUVA Sinus Implant becomes dislodged from the Delivery System prior to placement into the ethmoid sinus, remove the Implant and inspect for damage, re-load the undamaged Implant in the Crimper, and re-crimp the Implant into the Delivery System. Note that the SINUVA Sinus Implant should not be loaded into the Delivery System more than twice.
- Release the SINUVA Sinus Implant by pressing down on the Thumb Rest while pulling back on the Finger Rests in a controlled manner.
- Place the SINUVA Sinus Implant amongst the sinus polyps with the cap oriented toward the posterior ethmoid sinus, and with the Implant positioned as superiorly as possible in the sinus. The long ends of the Implant should be in approximately the 2 o'clock, 4 o'clock, 8 o'clock and 10 o'clock positions, respectively. Confirm final placement of the SINUVA Sinus Implant by endoscopic visualization. To adjust the position of the SINUVA Sinus Implant, use the Seeker on the Delivery System or standard endoscopic surgical instruments.
2.6 Removal Instructions
The SINUVA Sinus Implant is made from bioabsorbable polymers designed to gradually soften over time. The Implant may be left in the sinus to gradually release the corticosteroid over 90 days. The SINUVA Sinus Implant can be removed at any time at the physician's discretion using standard endoscopic instruments.
- 3 DOSAGE FORM AND STRENGTH
-
4 CONTRAINDICATIONS
Patients with known hypersensitivity to mometasone furoate, or to any of the copolymers of the SINUVA Sinus Implant [see Description (11)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Local Effects
Monitor nasal mucosa adjacent to the SINUVA Sinus Implant for any signs of bleeding (epistaxis), irritation, infection, or perforation. Avoid use in patients with nasal ulcers or trauma.
5.2 Ocular Effects
Glaucoma, cataracts, and clinically significant elevation of intraocular pressure were not observed in patients from the treatment group of one randomized controlled clinical study (N = 53) who underwent bilateral placement of SINUVA Sinus Implants. Close monitoring is warranted in patients with a change in vision or with a history of increased intraocular pressure, glaucoma, and/or cataracts.
5.3 Hypersensitivity Reactions
Hypersensitivity reactions, including rash, pruritus, and angioedema have been reported with use of corticosteroids.
5.4 Immunosuppression
Persons who are using drugs that suppress the immune system are more susceptible to infections than healthy individuals. Chickenpox and measles, for example, can have a more serious or even fatal course in susceptible children or adults using corticosteroids. In such children or adults who have not had these diseases or who are not properly immunized, particular care should be taken to avoid exposure. How the dose, route, and duration of corticosteroid administration affect the risk of developing a disseminated infection is not known.
The contribution of the underlying disease and/or prior corticosteroid treatment to the risk is also not known. If exposed to chickenpox, prophylaxis with varicella zoster immune globulin (VZIG) may be indicated. If exposed to measles, prophylaxis with pooled intramuscular immunoglobulin (IG) may be indicated (See the respective package inserts for complete VZIG and IG prescribing information). If chickenpox develops, treatment with antiviral agents may be considered. Corticosteroids should be used with caution, if at all, in patients with active or quiescent tuberculosis infection of the respiratory tract; untreated systemic fungal, bacterial, viral, or parasitic infections; or ocular herpes simplex.
5.5 Hypercorticism and Adrenal Suppression
Hypercorticism and adrenal suppression were not evaluated as part of the SINUVA Sinus Implant clinical program.
Since individual sensitivity to effects of cortisol production exists, physicians should consider this information when prescribing SINUVA Sinus Implant. Particular care should be taken in observing patients postoperatively or during periods of stress for evidence of inadequate adrenal response.
It is possible that systemic corticosteroid effects such as hypercorticism and adrenal suppression may appear in patients, particularly when systemic mometasone furoate is administered at higher than recommended doses over prolonged periods of time. If such effects occur, consider sinus implant removal.
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in more detail in other sections of the labeling:
- Local effects including epistaxis, irritation, infection, or perforation [see Warnings and Precautions (5.1)]
- Cataracts and glaucoma [see Warnings and Precautions (5.2)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.3)]
- Immunosuppression [see Warnings and Precautions (5.4)]
- Hypothalamic-pituitary-adrenal (HPA) axis effects [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of the SINUVA Sinus Implant was evaluated and demonstrated in 400 patients in 2 controlled, randomized, parallel group, single-blind studies. In Study 1, one-hundred (100) subjects were followed for 6 months. In Study 2, three-hundred (300) subjects were followed for 90 days. Of the 400 patients, 254 were assigned to the treatment group and underwent bilateral placement of SINUVA Sinus Implants in the ethmoid sinuses, totaling 2700 mcg of mometasone furoate, and 146 patients were assigned to the control group and underwent a sham procedure consisting of advancement of the Delivery System with the SINUVA Sinus Implant followed by removal without deployment. The Implants were removed by Day 60. All patients were required to use mometasone furoate nasal spray once daily (200 mcg of mometasone furoate) through Day 90.
Table 1 shows the common adverse reactions (in greater than 1% of subjects) that occurred more frequently in patients treated with SINUVA Sinus Implant compared to the control group.
Table 1: Adverse Reactions with > 1% Incidence and More Common than Control in 90-Day Controlled Clinical Trials with SINUVA Sinus Implant Study 1 & Study 2 Combined Data Adverse Reaction Treatment *
(N = 254)
n (%)Control †
(N = 146)
n (%)Values represent patient counts and percentages. A patient reporting more than one adverse event for a particular MedDRA preferred term is counted only once. - * Patients in the treatment group received SINUVA Sinus Implants placed bilaterally in the ethmoid sinuses and used mometasone furoate nasal spray once daily (200 mcg mometasone furoate) through Day 90.
- † Patients in the control group underwent a sham procedure and used mometasone furoate nasal spray once daily (200 mcg mometasone furoate) through Day 90.
Asthma 12 (4.7) 6 (4.1) Headache 9 (3.5) 5 (3.4) Epistaxis 6 (2.4) 2 (1.4) Presyncope 6 (2.4) 3 (2.1) Bronchitis 5 (2.0) 2 (1.4) Otitis media 5 (2.0) 2 (1.4) Nasopharyngitis 3 (1.2) 1 (0.7) Study 1 monitored patients from Day 90 through 6 months. Hypersensitivity (4% (n=2) vs. 0), chronic sinusitis (11% (n=6) vs. 9% (n=4)), and upper respiratory tract infections (8% (n=4) vs. 2% (n=1)) were reported in more than 2 subjects in the treatment group, and more commonly than the control group during this time period.
-
7 DRUG INTERACTIONS
Formal drug-drug interaction studies have not been conducted with the SINUVA Sinus Implant. An evaluation of the concurrent administration of the SINUVA Sinus Implant and other commonly used nasal drugs was not associated with any unusual adverse reactions.
7.1 Inhibitors of Cytochrome P450 3A4
Co-administration with ketoconazole, a potent CYP 3A4 inhibitor, may increase the plasma concentrations of mometasone furoate [see Clinical Pharmacology (12.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no randomized clinical studies of SINUVA Sinus Implant or mometasone furoate in pregnant women. The active pharmaceutical ingredient, mometasone furoate is systemically available when administered topically or when inhaled. In animal reproduction studies, subcutaneous administration of mometasone furoate to pregnant mice, rats, or rabbits caused increased fetal malformations and decreased fetal survival and growth following administration of doses that produced exposures approximately 1/3 to 8 times the maximum recommended human dose (MRHD) on a mcg/m2 or AUC basis [see Data]. However, experience with oral corticosteroids suggests that rodents are more prone to teratogenic effects from corticosteroid exposure than humans.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
In an embryofetal development study with pregnant mice dosed throughout the period of organogenesis, mometasone furoate produced cleft palate at an exposure approximately one-third of the MRHD (on a mcg/m2 basis with maternal subcutaneous doses of 60 mcg/kg and above) and decreased fetal survival at an exposure approximately equivalent to the MRHD (on a mcg/m2 basis with a maternal subcutaneous dose of 180 mcg/kg). No toxicity was observed with a dose that produced an exposure approximately one-tenth of the MRHD (on a mcg/m2 basis with maternal topical dermal doses of 20 mcg/kg and above).
In an embryofetal development study with pregnant rats dosed throughout the period of organogenesis, mometasone furoate produced fetal umbilical hernia at exposures approximately 6 times the MRHD (on a mcg/m2 basis with maternal topical dermal doses of 600 mcg/kg and above) and delays in fetal ossification at exposures approximately 3 times the MRHD (on a mcg/m2 basis with maternal topical dermal doses of 300 mcg/kg and above).
In another reproductive toxicity study, pregnant rats were dosed with mometasone furoate throughout pregnancy or late in gestation. Treated animals had prolonged and difficult labor, fewer live births, lower birth weight, and reduced early pup survival at an exposure that was approximately 8 times the MRHD (on an area under the curve (AUC) basis with a maternal subcutaneous dose of 15 mcg/kg). There were no findings with an exposure approximately 4 times the MRHD (on an AUC basis with a maternal subcutaneous dose of 7.5 mcg/kg).
Embryofetal development studies were conducted with pregnant rabbits dosed with mometasone furoate by either the topical dermal route or oral route throughout the period of organogenesis. In the study using the topical dermal route, mometasone furoate caused multiple malformations in fetuses (e.g., flexed front paws, gallbladder agenesis, umbilical hernia, hydrocephaly) at an exposure approximately 3 times the MRHD (on a mcg/m2 basis with maternal topical dermal doses of 150 mcg/kg and above). In the study using the oral route, mometasone furoate caused increased fetal resorptions and cleft palate and/or head malformations (hydrocephaly and domed head) at an exposure approximately 1/2 of the MRHD (on AUC basis with a maternal oral dose of 700 mcg/kg). At an exposure approximately 2 times the MRHD (on an AUC basis with a maternal oral dose of 2800 mcg/kg), most litters were aborted or resorbed. No effects were observed at an exposure approximately 1/10 of the MRHD (on an AUC basis with a maternal oral dose of 140 mcg/kg).
8.2 Lactation
Risk Summary
There are no available data on the presence of SINUVA Sinus Implant in human milk, the effects on the breastfed child or the effects on milk production. Systemic absorption of a single inhaled 400 mcg mometasone dose was less than 1%. It is not known if mometasone furoate is excreted in human milk. Other inhaled corticosteroids, similar to mometasone furoate, are present in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for the SINUVA Sinus Implant and any potential adverse effects on the breastfed infant from the SINUVA Sinus Implant.
8.4 Pediatric Use
The safety and effectiveness of the SINUVA Sinus Implant have not been established in children or adolescents less than 18 years of age.
8.5 Geriatric Use
A total of 33 patients 65 years of age or older received the SINUVA Sinus Implant in 2 controlled randomized clinical trials. Clinical studies did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects.
8.6 Hepatic Impairment
Concentrations of mometasone furoate appear to increase with severity of hepatic impairment [see Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
There are no data available on the effects of acute or chronic overdosage with SINUVA Sinus Implant. Chronic overdosage may result in signs/symptoms of hypercorticism [see Warnings and Precautions (5.5)].
Because the SINUVA Sinus Implant is designed for placement in the ethmoid sinus by a physician under endoscopic visualization, overdose is unlikely to occur. Low systemic bioavailability and an absence of acute drug-related systemic findings in clinical studies, indicate that acute overdose is unlikely to require any treatment other than observation. Single inhalation daily doses of a mometasone furoate dry powder inhalation formulation as high as 1200 mcg per day for 28 days were well tolerated and did not cause a significant reduction in plasma cortisol AUC (94% of placebo AUC). Single oral doses up to 8000 mcg have been studied on human volunteers with no adverse reactions reported.
-
11 DESCRIPTION
The SINUVA Sinus Implant is a self-expanding, bioabsorbable, drug eluting implant provided with a crimper and a single-use delivery system. SINUVA Sinus Implant is comprised of poly(L-lactide-co-glycolide) and poly(L-lactide-co-ε-caprolactone) coated with mometasone furoate embedded in a bioabsorbable polymer matrix containing poly(DL-lactide-co-glycolide) and polyethylene glycol (inactive ingredients) which provides for gradual release of the drug. The SINUVA Sinus Implant is packaged in a tray, which is then sealed in a foil pouch and placed in the product carton. The SINUVA Sinus Implant is provided sterile.
Mometasone furoate, the active component of the SINUVA Sinus Implant, is a corticosteroid with the chemical name 9,21-dichloro-11(β),17-dihydroxy-16(α)-methylpregna-1,4-diene-3,20-dione 17 (2-furoate). Mometasone furoate is a white powder with an empirical formula of C27H30Cl2O6, and molecular weight of 521.44 Daltons.
The chemical structure of mometasone furoate is shown below:
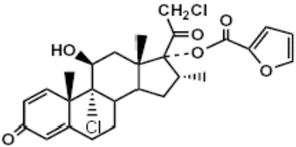
The inactive ingredients are poly-(DL-lactide-co-glycolide) and polyethylene glycol. Poly-(DL-lactide-co-glycolide) is an amorphous biodegradable polymer.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Mometasone furoate is a corticosteroid demonstrating potent anti-inflammatory activity. The precise mechanism of corticosteroid action on inflammation is not known. Corticosteroids have been shown to have a wide range of effects on multiple cell types (e.g., mast cells, eosinophils, neutrophils, macrophages, and lymphocytes) and mediators (e.g., histamine, eicosanoids, leukotrienes, and cytokines) involved in inflammation.
12.3 Pharmacokinetics
One pharmacokinetics study was conducted with the SINUVA Sinus Implant. The remaining information is from other products containing mometasone furoate.
Absorption:
A pharmacokinetics study was conducted to evaluate the potential for systemic exposure to mometasone furoate from the sinonasal route of administration following bilateral placement of the SINUVA Sinus Implant. Baseline blood samples were taken before the procedure, and on Days 3, 7, 14, 21 and 30 to assess systemic concentrations of mometasone furoate in plasma. Six out of fifteen PK samples from five subjects had measurable mometasone furoate plasma concentrations from Day 3 to Day 14. All the measurable concentrations were within 2.5-fold of the lower limit of quantitation (LLOQ; 30 pg/mL). No PK samples had measurable mometasone furoate plasma concentrations after Day 14.
Distribution:
Following an intravenous 400 mcg dose of mometasone furoate, the mean steady state volume of distribution was 152 L. The in vitro protein binding for mometasone furoate was reported to be 98% to 99% (in concentration range of 5 to 500 ng/mL).
Metabolism:
Studies have shown that mometasone furoate is primarily and extensively metabolized in the liver of all species investigated and undergoes extensive metabolism to multiple metabolites. In vitro studies have confirmed the primary role of CYP 3A4 in the metabolism of this compound; however, no major metabolites were identified.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 2-year carcinogenicity study in Sprague Dawley rats, mometasone furoate demonstrated no statistically significant increase of tumors at inhalation doses up to 67 mcg/kg (approximately 14 times the MRHD on an AUC basis). In a 19-month carcinogenicity study in Swiss CD-1 mice, mometasone furoate demonstrated no statistically significant increase in the incidence of tumors at inhalation doses up to 160 mcg/kg (approximately 9 times the MRHD on an AUC basis).
Mometasone furoate increased chromosomal aberrations in an in vitro Chinese hamster ovary cell assay, but did not have this effect in an in vitro Chinese hamster lung cell assay. Mometasone furoate was not mutagenic in the Ames test or mouse lymphoma assay, and was not clastogenic in an in vivo mouse micronucleus assay, a rat bone marrow chromosomal aberration assay, or a mouse male germ-cell chromosomal aberration assay. Mometasone furoate also did not induce unscheduled DNA synthesis in vivo in rat hepatocytes.
In reproductive studies in rats, impairment of fertility was not produced by subcutaneous doses up to 15 mcg/kg (approximately 8 times the MRHD on an AUC basis).
-
14 CLINICAL STUDIES
The safety and efficacy of the SINUVA Sinus Implant was evaluated in 400 patients, 18 years of age and older, with nasal polyps and a history of ethmoid sinus surgery. The development program included a trial of 6 months duration (Study 1: NCT01732536) and another trial of 90 days duration (Study 2: NCT02291549). The efficacy of SINUVA Sinus Implant is based primarily on Study 2 as described below.
Study 2 was a randomized, controlled, single-blind, multicenter (all sites were in the US) study with 300 patients: 201 patients were assigned to the treatment group and underwent bilateral placement of the SINUVA Sinus Implants in the ethmoid sinuses. The remaining 99 patients were assigned to the control group and underwent a placebo (sham) procedure, consisting of advancement of the Delivery System with the SINUVA Sinus Implant into the ethmoid sinuses, followed by removal of the Delivery System without deployment of the SINUVA Sinus Implant. The Implants were removed by Day 60 to allow blinded grading at Day 90. All patients [treatment (T) and control (C) groups] were required to use a mometasone furoate nasal spray once daily (200 mcg of mometasone furoate) through Day 90.
The co-primary efficacy endpoints were:
- Change from baseline to Day 30 in Nasal Obstruction/Congestion score, as determined by patients using a daily diary; and
- Change from baseline to Day 90 in bilateral polyp grade, as determined from video-endoscopies reviewed by an independent panel of 3 sinus surgeons who were masked to treatment assignment.
The study population consisted of adult patients (≥ 18 years of age) diagnosed with chronic sinusitis who had undergone prior bilateral total ethmoidectomy, but were indicated for revision endoscopic sinus surgery because they presented with recurrent nasal obstruction/congestion symptoms and recurrent bilateral sinus obstruction due to sinonasal polyposis. Subjects were excluded for other grade 3 or 4 adhesions/synechiae, grade 4 polyposis, known history of resistant or poor response to oral steroids, acute bacterial or invasive fungal sinusitis, and immune deficiency, including cystic fibrosis. There were no statistically significant differences between groups in baseline demographics and clinical characteristics, except the treatment group had a higher proportion of asthma patients (T: 74% vs. C: 62%) and higher mean Percent Ethmoid Sinus Obstruction score [T: 76 (SD 17.4) vs. C: 69 (SD 19.9)]. The random imbalances did not impact treatment effect.
The co-primary efficacy results are presented in Table 2. The treatment group demonstrated a statistically significant difference from baseline to Day 30 in Nasal Obstruction/Congestion score and from baseline to Day 90 in bilateral polyp grade, compared to the control group.
Table 2: Co-Primary Efficacy Results with the SINUVA Sinus Implant (Study 2) Treatment
(N = 201)Control
(N = 99)- * Change from baseline in Nasal Obstruction/Congestion was assessed on a scale of 0–3 where 0=no symptoms, 1=mild symptoms, 2=moderate symptoms, and 3=severe symptoms. Scores were assessed using a daily diary for 7 days immediately preceding the baseline and Day 30 visits.
- † Based on analysis of covariance (ANCOVA) model with baseline value as a covariate and site and treatment group as fixed effects.
- ‡ Change from baseline to Day 90 in bilateral polyp grade was assessed based on grading of video-endoscopies by an independent panel of 3 sinus surgeons who were blinded to treatment assignment. Polyps were graded as follows: 0=no visible polyps, 1=Small amount of sinonasal polyps confined in middle meatus, 1.5= 1+ polypoid edema obstructing ≥ 25% of the ethmoid sinus cavity, 2=Expanded amount of sinonasal polyps confined in middle meatus, 2.5 = 2 + polypoid edema obstructing ≥ 50% of the ethmoid sinus cavity, 3=Sinonasal polyps extending beyond middle meatus, but not totally obstructing the nasal cavity, 3.5= 3 + polypoid edema obstructing ≥ 75% of the ethmoid sinus cavity, 4=Sinonasal polyps completely obstructing the nasal cavity.
Nasal Obstruction/Congestion Score* N 201 99 Baseline, Mean (SD) 2.36 (0.49) 2.35 (0.48) Change from Baseline, Mean (SD) -0.80 (0.73) -0.56 (0.62) Difference vs. Control (95% CI)† -0.23 (-0.39, -0.06) Bilateral Polyp Grade‡ N 195 97 Baseline, Mean (SD) 5.48 (1.13) 5.43 (1.01) Change from Baseline, Mean (SD) -0.56 (1.06) -0.15 (0.91) Difference vs. Control (95% CI)† -0.35 (-0.60, -0.09) Change from baseline to Day 90 in the mean Percent Ethmoid Sinus Obstruction score (100 mm VAS), as judged by the independent panel [Difference vs. control: -7.96%; 95% CI (-12.1, -3.8)], met statistical significance and supported the co-primary endpoints.
- 16 HOW SUPPLIED/STORAGE AND HANDLING
-
17 PATIENT COUNSELING INFORMATION
- Encourage patients to use saline irrigations or sprays regularly.
- Advise the patient that the Implant is bioabsorbable and intended to soften over time. As the Implant softens and polyps decrease, the Implant may be expelled out of the nose on its own or with actions such as sneezing or forceful nose blowing.
- Advise the patient to call a health care professional immediately if they experience any of the following:
- Excessive nasal bleeding or symptoms of infection, such as excessive pain or discomfort, persistent headache, increased sinus discharge.
- Symptoms suggesting the Implant has migrated posteriorly, such as irritation or choking sensation in the back of the throat.
17.1 Risks Relating to the Insertion and Removal Procedure
Inform patients that there are risks associated with the insertion and removal of the SINUVA Sinus Implant. These risks are similar to those associated with other endoscopic sinus procedures.
17.2 Local Effects
Patients should be informed that treatment with the SINUVA Sinus Implant may be associated with local adverse reactions such as nose bleed (epistaxis), injury to nerves or blood vessels of the middle turbinate or septum, bacterial or candida infection. Because of the potential inhibitory effect of corticosteroids on wound healing, patients who have experienced recent nasal septum ulcers, nasal surgery, or nasal trauma should not use a nasal corticosteroid until healing has occurred [see Warnings and Precautions (5.1) and Adverse Reactions (6)].
17.3 Hypersensitivity Reactions
Hypersensitivity reactions, including rash, pruritus, and angioedema, have been reported with use of mometasone furoate. Remove the SINUVA Sinus Implant if such reactions occur [see Contraindications (4), Warnings and Precautions (5.3), and Adverse Reactions (6)].
17.4 Immunosuppression
Patients who are on immunosuppressant doses of corticosteroids should be warned to avoid exposure to chickenpox or measles and, if exposed, to consult their physician without delay. Patients should be informed of potential worsening of existing tuberculosis; fungal, bacterial, viral, or parasitic infections; or ocular herpes simplex [see Warnings and Precautions (5.4)].
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 1350 mcg Implant Pouch Carton
-
INGREDIENTS AND APPEARANCE
SINUVA
mometasone furoate implantProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 10599-003 Route of Administration INTRASINAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MOMETASONE FUROATE (UNII: 04201GDN4R) (MOMETASONE - UNII:8HR4QJ6DW8) MOMETASONE FUROATE 1350 ug Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 10599-003-01 1 in 1 CARTON 02/01/2018 1 1 in 1 POUCH; Type 4: Device Coated/Impregnated/Otherwise Combined with Drug Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA209310 02/01/2018 Labeler - Intersect ENT, Inc. (876715355) Establishment Name Address ID/FEI Business Operations Intersect ENT, Inc. 876715355 MANUFACTURE(10599-003)
Trademark Results [Sinuva]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SINUVA 88370241 5879694 Live/Registered |
Intersect ENT Inc. 2019-04-03 |
 SINUVA 87067979 5459633 Live/Registered |
Intersect ENT Inc. 2016-06-10 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
