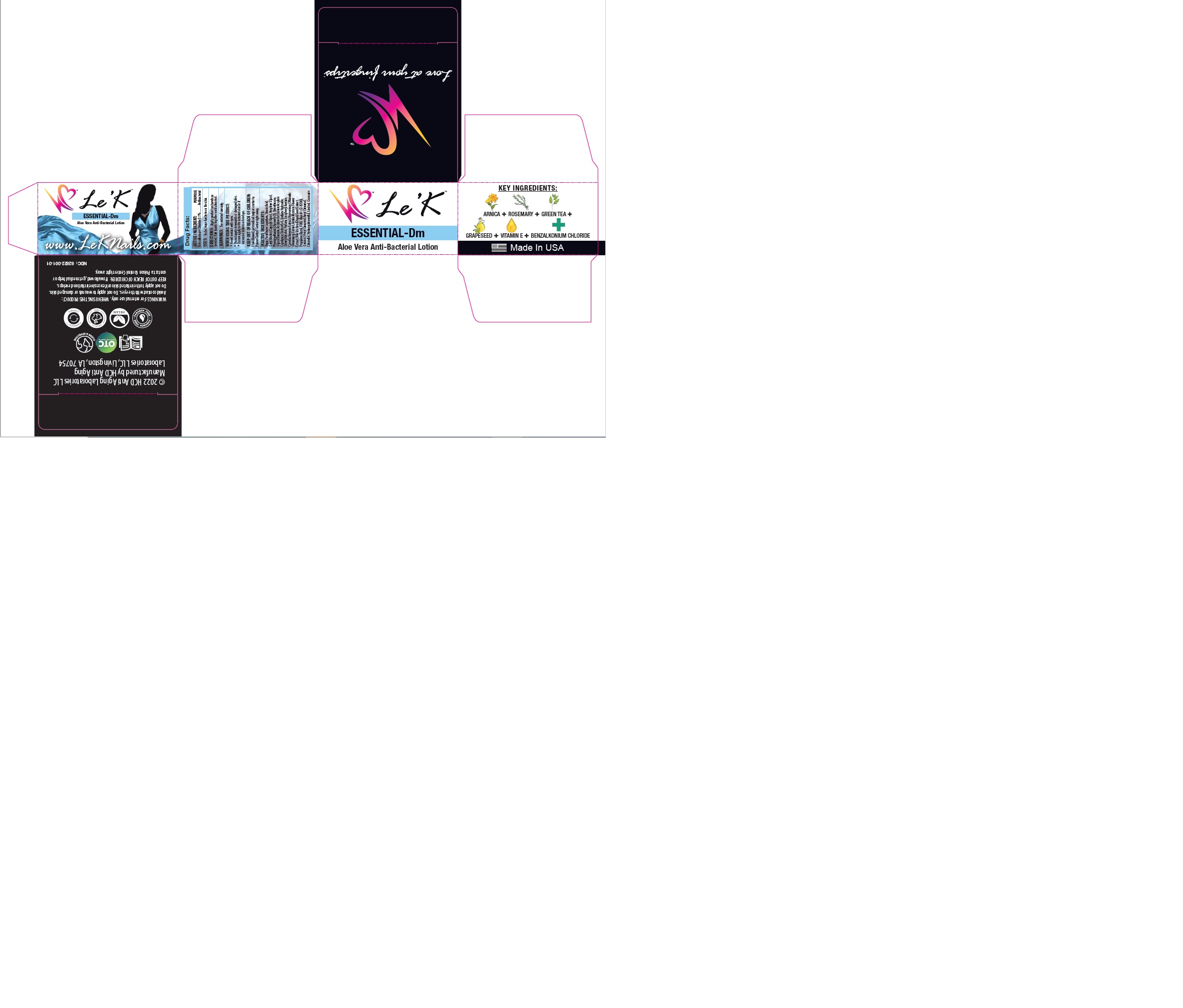
Apply product on hands as needed. Rub hands together until absorbed.
Aqua, Paraffinum Liquidum, Stearic Acid, Cetyl Alcohol, Caprylyl Glycol, Hexylene Glycol, Phenoxyethanol, Glycereth-26, Dimethicone, Glycol Stearate, Acrylamide/Sodium Acrylate Copolymer, Trideceth-6, Sodium Hydroxide, Stearyl Alcohol, Parfum, Tocopheryl Acetate, Camelia Sinensis Extract, Rosmarinus Officinalis (Rosemary) Extract, Arnica Montana Flower Extract, Vitis Vinifera (Grape) Seed Extract, Propylene Glycol, FD&C Blue #1 (CI 42090), Geraniol, Hexyl Cinnamal, Amyl Cinnamal, Citronellol, Hydroxycitronnellal, Linalool, Coumarin.
To decrease bacteria on the skin.
If swallowed, get medical help or contact a Poison Control Center right away.
Benzalkonium chloride 0.1%………………….….…………………………………………………………Antibacterial
Benzalkonium chloride 0.1%