TOLAK- fluorouracil cream
TOLAK by
Drug Labeling and Warnings
TOLAK by is a Prescription medication manufactured, distributed, or labeled by Pierre Fabre Pharmaceuticals, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use TOLAK CREAM safely and effectively. See full prescribing information for TOLAK CREAM.
TOLAK (fluorouracil) Cream, 4%, for topical use
Initial U.S. Approval: 1962INDICATIONS AND USAGE
Tolak (fluorouracil) Cream, 4%, is a nucleoside metabolic inhibitor indicated for the topical treatment of actinic keratosis lesions of the face, ears, and scalp ( 1).
DOSAGE AND ADMINISTRATION
- Apply Tolak Cream after washing, rinsing, and drying the treatment area(s) ( 2).
- Apply Tolak Cream once daily in an amount sufficient to cover the lesions of the face, ears, and/or scalp with a thin film, using the fingertips to gently massage the medication uniformly into the skin ( 2).
- Tolak Cream should be applied for a period of 4 weeks as tolerated ( 2).
- Wash hands thoroughly following Tolak Cream application ( 2).
- Tolak Cream is for topical use only. Do not apply to eyes, nose, mouth or mucous membranes ( 2).
- Not for ophthalmic, oral, or intravaginal use ( 2).
DOSAGE FORMS AND STRENGTHS
Cream: 40 mg of fluorouracil per gram of cream (4%) ( 3).
WARNINGS AND PRECAUTIONS
- Application site adverse reactions are likely to occur during and for 4 weeks after treatment of actinic keratosis with Tolak Cream ( 5.1).
- Hypersensitivity reactions may occur with Tolak Cream ( 5.2).
- Avoid treatment in the periocular area. Eye disorders, including corneal reactions have occurred with topical fluorouracil use. Avoid accidental transfer of the drug into eyes and to the periocular area. If accidental exposure occurs, seek medical care ( 5.3).
- Increased sensitivity to ultraviolet light may occur during and immediately after treatment with Tolak Cream ( 5.4).
- Fluorouracil may cause fetal harm when administered to a pregnant woman. Advise females of reproductive potential to use effective contraception ( 5.5).
- Increased fluorouracil exposures may occur in DPD deficiency. Discontinue Tolak Cream if symptoms of fluorouracil's systemic toxicity develop ( 5.6).
ADVERSE REACTIONS
The most common (incidence > 68%) adverse reactions occur at the application site and include erythema, scaling/dryness, crusting, pruritus, stinging/burning, edema, and erosions. Erythema is observed in 99% of patients with actinic keratosis treated with Tolak Cream ( 6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Pierre Fabre Pharmaceuticals at 1-855-MYTOLAK (698-6525) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 3/2017
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Pregnancy
4.2 Dihydropyrimidine Dehydrogenase Deficiency
5 WARNINGS AND PRECAUTIONS
5.1 Application Site Adverse Reactions
5.2 Hypersensitivity Reactions
5.3 Ophthalmic Adverse Reactions
5.4 Photosensitivity
5.5 Embryofetal Toxicity
5.6 Toxicity in Patients with Dihydropyrimidine Dehydrogenase Deficiency
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED / STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
Prior to application of Tolak Cream, wash, rinse, and dry the treatment areas. Apply Tolak Cream once daily in an amount sufficient to cover the lesions of the face, ears, and/or scalp with a thin film, using the fingertips to gently massage the medication uniformly into the skin. Apply Tolak Cream for a period of 4 weeks as tolerated. Thoroughly wash hands following Tolak Cream application.
Tolak Cream is for topical use only. Do not apply to eyes, nose, mouth or mucous membranes. Not for ophthalmic, oral or intravaginal use.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Tolak Cream is contraindicated:
- During pregnancy [see Warnings and Precautions (5.5, 8.1)]
- In patients with dihydropyrimidine dehydrogenase (DPD) deficiency [see Warnings and Precautions (5.6)]
-
5 WARNINGS AND PRECAUTIONS
5.1 Application Site Adverse Reactions
Application site reactions (erythema, scaling/dryness, edema, crusting, erosions, stinging/burning, and pruritus) were observed in almost all patients during treatment of actinic keratosis on the face, ears, and/or scalp with topical fluorouracil [see Adverse Reactions (6.1)] . In the clinical trials of Tolak Cream, application site irritation returned to baseline (pre-treatment) levels within 4 weeks after discontinuing treatment.
Do not apply Tolak Cream directly into eyes, nose, mouth, or other mucous membranes because irritation, local inflammation and ulceration can occur.
5.2 Hypersensitivity Reactions
Allergic contact dermatitis (delayed type hypersensitivity reaction) has been noted for topical fluorouracil drugs. While application site reactions are observed in almost all patients during treatment of actinic keratosis with topical fluorouracil [see Adverse Reactions (6.2)] , delayed type hypersensitivity should be suspected in the event of severe pruritus or eczema at the application site or at a distant site. Although the potential for a delayed hypersensitivity reaction to fluorouracil exists, patch testing to confirm hypersensitivity may be inconclusive.
Tolak Cream contains peanut oil. If signs of hypersensitivity occur, patients should discontinue Tolak Cream immediately and contact their healthcare provider.
5.3 Ophthalmic Adverse Reactions
Corneal and conjunctival disorders have occurred with topical fluorouracil use [see Adverse Reactions (6.2)] . Avoid application to the periocular area. To avoid transfer of the drug into the eyes and to the periocular area during and after application, patients should wash hands well after applying Tolak Cream. If accidental exposure occurs, the patient should flush eye(s) with large amounts of water and seek medical care as soon as possible.
5.4 Photosensitivity
Topical fluorouracil is associated with photosensitivity reactions including severe sunburn. Minimize exposure to ultraviolet rays including sunlight, sun lamps, and tanning beds during and immediately following treatment with Tolak Cream because the intensity of the photosensitivity reaction may be increased.
5.5 Embryofetal Toxicity
Cases of miscarriage and birth defects (including cleft lip and cleft palate) have been reported when pregnant women were exposed to a topical or parenteral fluorouracil product. In addition, ventricular septal defect and cases of miscarriage occurred when pregnant women applied a topical fluorouracil product to mucous membranes (Tolak Cream is not indicated for use on the mucous membrane). Furthermore, fluorouracil interferes with the synthesis of deoxyribonucleic acid (DNA), inhibits the formation of ribonucleic acid (RNA), and provokes unbalanced growth and death of cells. Therefore, Tolak Cream is contraindicated in pregnancy.
Advise females of reproductive potential to use effective contraception during Tolak use and for one month after the last dose of Tolak Cream .
5.6 Toxicity in Patients with Dihydropyrimidine Dehydrogenase Deficiency
Life-threatening systemic toxicity has been reported with the topical use of fluorouracil in a patient with DPD deficiency. Symptoms included severe abdominal pain, bloody diarrhea, vomiting, fever, and chills. Physical examination revealed stomatitis, erythematous skin rash, neutropenia, thrombocytopenia, inflammation of the esophagus, stomach and small bowel.
A large percentage of fluorouracil is catabolized by the DPD enzyme. DPD enzyme deficiency may result in increased availability of fluorouracil to the anabolic pathway, which may lead to increased interference with DNA and RNA synthesis and increased cytotoxic activity and potential toxicities [see Clinical Pharmacology (12.1)] . Therefore, Tolak Cream is contraindicated in patients with DPD deficiency.
Patients should discontinue Tolak Cream if symptoms of fluorouracil's systemic toxicity develop.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed in more detail in other sections of the labeling:
- Application Site Adverse Reactions [see Warnings and Precautions (5.1)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.2)]
- Ophthalmic Adverse Reactions [see Warnings and Precautions (5.3)]
- Photosensitivity [see Warnings and Precautions (5.4)]
- Embryofetal toxicity [see Warnings and Precautions (5.5)]
- Toxicity in Patients with Dihydropyrimidine Dehydrogenase Deficiency [see Warnings and Precautions (5.6)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described below reflect exposure to Tolak Cream in 397 subjects with actinic keratosis in vehicle-controlled trials. The population ranged in age from 33 to 94 years, was 80% male, and almost all were Caucasian. Most subjects were treated with Tolak Cream once daily for 4 weeks. Throughout the 4-week treatment and the 4-week post-treatment periods, the trials specifically monitored for adverse reactions related to tolerability, including erythema, scaling/dryness, edema, crusting, erosions, stinging/burning, and pruritus.
The number and percentage of subjects with each of these monitored adverse reactions at one or more post-baseline visit(s) during the clinical trials are shown in Table 1.
Table 1: Incidence of Application Site Adverse Reactions Occurring with 4 Weeks of Tolak Cream Treatment in Clinical Trials 1 and 2 Tolak Cream
N=397
n (%)Vehicle
N=120
n (%)Mild, Moderate or Severe Severe Only Mild, Moderate or Severe Severe Only Erythema 394 (99%) 174 (44%) 102 (85%) 0 (0%) Scaling/ Dryness 377 (95%) 94 (24%) 99 (83%) 0 (0%) Crusting 346 (87%) 87 (22%) 46 (38%) 0 (0%) Pruritus 337 (85%) 65 (16%) 46 (38%) 1 (1%) Stinging/ Burning 346 (87%) 101 (25%) 42 (35%) 0 (0%) Edema 275 (69%) 30 (8%) 11 (9%) 0 (0%) Erosions 271 (68%) 44 (11%) 14 (12%) 0 (0%) In these clinical trials, the intensity of the adverse reactions in subjects using Tolak Cream generally increased over the 4-week treatment period, usually reaching maximal levels at 4 weeks of treatment and then diminishing to baseline levels within 4 weeks after cessation of treatment.
In Trials 1 and 2, 11% of Tolak Cream-treated and 3% of vehicle-treated subjects discontinued treatment because of adverse reactions. Of these subjects, the majority had adverse reactions at the application site. Eye swelling, leading to discontinuation, occurred in one subject with Tolak Cream use .
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of topical fluorouracil. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and Lymphatic System Disorders: leukocytosis, pancytopenia, thrombocytopenia, eosinophilia, neutrophil toxic granulation
Eye disorders: corneal disorder, conjunctival disorder, eye irritation, conjunctivitis, lacrimation
Gastrointestinal disorders: stomatitis
General Disorders and Administration Site Conditions: medicinal taste
Infections and Infestations: herpes simplex
Neoplasms: chronic lymphocytic leukemia, non-melanoma skin cancer
Nervous system disorders: insomnia, irritability
Psychiatric disorders: emotional distress
Skin and subcutaneous tissue disorders: blistering, allergic contact dermatitis, photosensitivity, pain, scarring, skin irritation, rash, ulceration, hyperpigmentation, alopecia, bullous pemphigoid, ichthyosis, suppuration, swelling, soreness, telangiectasia, tenderness, urticaria
-
7 DRUG INTERACTIONS
Subjects using systemic steroids, immunosuppressants, and immunomodulators were generally excluded from the clinical studies of Tolak Cream, as were subjects who used retinoids, topical steroids, glycolic acid products, alpha-hydroxy products, and chemical peeling products in the treatment areas. No clinical trials were designed to specifically evaluate drug interactions.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects: Pregnancy Category X [see Contraindications (4.1)] .
Cases of miscarriage and birth defects (including cleft lip and cleft palate) have been reported when pregnant women were exposed to a topical or parenteral fluorouracil product. In addition, ventricular septal defect and cases of miscarriage occurred when pregnant women applied a topical fluorouracil product to mucous membranes (Tolak Cream is not indicated for use on the mucous membrane).
Animal reproduction studies have not been conducted with Tolak Cream. Fluorouracil administered parenterally has been shown to be teratogenic in mice, rats, and hamsters when given at doses equivalent to the usual human intravenous dose. However, the amount of fluorouracil absorbed systemically after topical administration to actinic keratosis is minimal [see Clinical Pharmacology (12.3)] . Fluorouracil exhibited maximum teratogenicity when given to mice as single intraperitoneal injections of 10 to 40 mg/kg on day 10 or 12 of gestation. Similarly, intraperitoneal doses of 12 to 37 mg/kg given to rats between days 9 and 12 of gestation and intramuscular doses of 3 to 9 mg/kg given to hamsters between days 8 and 11 of gestation were teratogenic and/or embryotoxic (i.e., resulted in increased resorptions or embryolethality). In monkeys, divided doses of 40 mg/kg given between days 20 and 24 of gestation were not teratogenic. However, doses higher than 40 mg/kg resulted in spontaneous abortions. Based on the recommended human dose and instructions for use, it is not possible to calculate human dose equivalents for animal exposures in these studies.
8.3 Nursing Mothers
Because many drugs are excreted in human milk and there is some systemic absorption of fluorouracil after topical administration, and because of the potential for serious adverse reactions in nursing infants, a decision should be made whether to discontinue nursing or to discontinue drug use, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
Actinic keratosis is not usually observed in the pediatric population except in the case of rare genetic diseases. Tolak Cream is not intended for use in pediatric patients. Safety and effectiveness in children have not been established.
8.5 Geriatric Use
No dose adjustment is required for elderly patients [see Clinical Studies (14)] . The mean age of the 403 subjects treated with Tolak Cream in the clinical trials was 68 years. Of the Tolak Cream-treated subjects, 61% were 65 and over, while 28% were 75 and over.
No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
-
11 DESCRIPTION
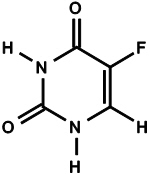
Tolak (fluorouracil) Cream, 4% contains 40 mg of fluorouracil per gram of white cream for topical application. It is a nucleoside metabolic inhibitor. Chemically, fluorouracil is 5-fluoro-2,4 (1H,3H)-pyrimidinedione. The molecular formula of 5-fluorouracil is C 4H 3FN 2O 2, and its molecular weight is 130.1. Its structural formula is:
Tolak Cream contains the following inactive ingredients: arlacel-165, butylated hydroxytoluene, cetyl alcohol, anhydrous citric acid, glycerin, isopropyl myristate, methyl gluceth-10, methylparaben, propylparaben, purified water, peanut oil, sodium hydroxide, stearic acid, and stearyl alcohol. Tolak Cream formulation has an alkaline pH at 8.3 to 9.2.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
There is evidence that the metabolism of fluorouracil in the anabolic pathway blocks the methylation reaction of deoxyuridylic acid to thymidylic acid. In this manner, fluorouracil interferes with the synthesis of DNA and to a lesser extent inhibits the formation of RNA. Since DNA and RNA are essential for cell division and growth, the effect of fluorouracil may be to create a thymine deficiency that provokes unbalanced growth and death of the cell. The effects of DNA and RNA deprivation are most marked on those cells that grow more rapidly and take up fluorouracil at a more rapid rate.
12.3 Pharmacokinetics
A systemic absorption study of topically applied Tolak Cream was performed in 21 patients with at least 3 actinic keratosis lesions (4 mm or greater in diameter). The steady state concentration of 5-fluorouracil in plasma was examined at 1, 2, 4, 6, 8, 10, 12, 16, and 24 hours after the last dose of a 4-week regimen in subjects with actinic keratosis after "area application" to area(s) in which actinic keratosis lesions were identified at baseline. Areas were defined as the whole region of the left cheek, right cheek, chin and forehead, bald scalp, and right and left ears, where actinic keratosis was identified at baseline. Thus, for example, if an actinic keratosis lesion was identified on the left cheek, Tolak Cream was to be applied as a thin film to the whole area of the left cheek.
Eight patients had undetectable levels of plasma 5-fluorouracil (the lower limit of quantification was 1.00 ng/ml) in all plasma samples following treatment with Tolak Cream. Among patients with detectable plasma 5-fluorouracil levels, the highest level of plasma 5-fluorouracil was generally observed at 1 hour post-dose. The mean observed maximum concentration (± standard deviation) of plasma 5-fluorouracil was 3.66 (±1.58) ng/mL with the range between 1.11 – 7.35 ng/mL.
The catabolism of 5-fluorouracil results in inactive degradation products (such as CO 2, urea, α-fluoro-β-alanine).
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Adequate long-term studies in animals to evaluate carcinogenic potential of fluorouracil have not been conducted. Studies with the active ingredient of Tolak, fluorouracil, have shown mutagenic effects in in vitro and in vivo tests and impairment of fertility in in vivo animal studies.
Fluorouracil was positive in three in vitro cell neoplastic transformation assays. In the C3H/10T½ clone 8 mouse embryo cell system, the resulting morphologically transformed cells formed tumors when inoculated into immunosuppressed syngeneic mice.
Although no evidence for mutagenic activity of fluorouracil was observed in 3 studies utilizing the Ames test, mutagenic activity was observed in the survival count rec-assay with Bacillus subtilis and in the Drosophila wing-hair spot test. Fluorouracil produced petite mutations in Saccharomyces cerevisiae and demonstrated positive results in the micronucleus test using bone marrow cells of male mice.
Fluorouracil demonstrated clastogenic activity in vitro in Chinese hamster fibroblasts at concentrations of 1.0 and 2.0 µg/mL and was associated with chromatid gaps, breaks, and exchanges. In human lymphocytes, fluorouracil increased sister chromatid exchange in vitro. Additionally, an increase in numerical and structural chromosome aberrations have been observed in peripheral lymphocytes of patients treated with 5-fluorouracil.
In rats, chromosomal abnormalities and changes in chromosome organization in spermatogonia have been observed after intraperitoneal administration of 125 to 250 mg/kg of fluorouracil. Spermatogonial differentiation was also inhibited and resulted in transient infertility. Fluorouracil was inactive, however, at oral doses of 5 to 80 mg/kg/day in studies with a strain of mouse which is sensitive to the induction of sperm head abnormalities after exposure to a range of chemical mutagens and carcinogens. In female rats, fluorouracil administered intraperitoneally at doses of 25 and 50 mg/kg during the preovulatory phase of oogenesis resulted in a significant reduction in the incidence of fertile matings, a delay in the development of preimplantation and postimplantation embryos, an increased incidence of preimplantation lethality, and an induction of chromosomal anomalies in these embryos. In mice, single intravenous or intraperitoneal injections of fluorouracil were toxic to differentiated spermatogonia and spermatocytes (at 500 mg/kg) and produced abnormalities in spermatids (at 50 mg/kg).
-
14 CLINICAL STUDIES
The efficacy and safety of Tolak Cream was evaluated in two double-blind multi-center trials (Trial 1 and Trial 2) in subjects with at least 5 visible actinic keratosis lesions on the face, scalp, and/or ears. Subjects applied the assigned medication (Tolak Cream or vehicle placebo) to the face, and/or ears and/or scalp once or twice daily for four weeks as directed. Application of the medication involved field treatment of the whole area of the face and/or ears and/or scalp where actinic keratosis lesions were identified at baseline. Subjects receiving confounding treatments or medications were excluded. The effect of treatment was assessed at 4 weeks post-treatment. Subjects were almost all Caucasian, the mean age was approximately 68 years (range was from 33 to 89 years), and the mean number of actinic keratosis lesions was 14.4 in the Tolak group and 16.2 in the vehicle group in Trial 1, and 19.2 in the Tolak group and 23.2 in the vehicle group in Trial 2.
The number and percentage of subjects with 100% clearing of their actinic keratosis lesions and with at least 75% clearing of their actinic keratosis lesions are shown in Table 2.
Table 2 Subjects with 100% and at least 75% Clearing of Actinic Keratosis Lesions at 4 Weeks Post-Treatment Tolak Cream
% (n/N)Vehicle
% (n/N)Subjects with 100% Clearing of Actinic Keratosis Lesions Trial 1 54% (192/353) 4% (3/70) Trial 2 24% (12/50) 4% (2/50) Subjects with At Least 75% Clearing of Actinic Keratosis Lesions Trial 1 80% (284/353) 7% (5/70) Trial 2 74% (37/50) 10% (5/50) Examination of age (< 68 years versus ≥ 68 years) and gender subgroups did not identify differences in response to Tolak Cream among these subgroups. There were too few non-Caucasian subjects to adequately assess differences in effects among racial subgroups.
After completing Trials 1 and 2, subjects who achieved 100% clearing of actinic keratosis lesions with Tolak Cream treatment were followed for 12 months for lesion recurrence. Table 3 presents the long term outcomes of these 204 subjects.
Table 3 Recurrence of Actinic Keratosis Lesions within 12 Months After Completing Trial 1 or 2 Cleared Tolak Subjects
N=204- * Subjects who applied other treatments for actinic keratosis were counted as having recurrence.
Subjects remained clear 12 months after treatment 56 (27%) Subjects with recurrence within 12 months * 110 (54%) Subjects with no follow-up 38 (19%) -
16 HOW SUPPLIED / STORAGE AND HANDLING
16.1 How Supplied
Tolak (fluorouracil) Cream, 4% containing 40 mg of fluorouracil per gram of white cream is available in a 40 gram tube (NDC: 64370-421-40).
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Important Administration Instructions
Advise patients of the following:
- Tolak Cream is for external use only.
- Do not apply to eyes, nose, mouth or mucous membranes.
- Avoid inadvertent transfer of Tolak Cream to other body areas, or to another person.
- Keep out of the reach of children.
Instruct patients to do the following:
- Apply after washing, rinsing, and drying the treatment area.
- Wash hands thoroughly after application.
Hypersensitivity Reactions
- Inform patients that Tolak Cream contains peanut oil and that hypersensitivity reactions may occur with its use.
- Inform patients to discontinue Tolak Cream immediately and seek medical attention if signs of severe hypersensitivity occur [see Warnings and Precautions (5.2)] .
Ophthalmic Adverse Reactions
- Inform patients that ophthalmic adverse reactions can occur with Tolak Cream use.
- Advise patients that Tolak Cream is not for ophthalmic use.
- Advise patients to avoid application around the eyes.
- If accidental exposure occurs, advise patients to flush eye(s) with large amounts of water and seek medical care [see Warnings and Precautions (5.3)] .
Increased Sensitivity to UV Light
- Inform patients that topical fluorouracil is associated with photosensitivity reactions including severe sunburn.
- Advise patients to minimize exposure to sun, sun lamps, and tanning beds while using Tolak Cream.
- Advise patients that sunscreens may be applied after Tolak Cream application [see Warnings and Precautions (5.4)] .
Embryofetal Toxicity
- Inform females of reproductive potential of the potential risk to a fetus.
- Advise females of reproductive potential to use effective contraception during and for one month after the last dose of Tolak Cream and to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.5)].
Systemic Toxicity in Patients with DPD deficiency
- Advise patient to stop using Tolak immediately and contact physician if abdominal (stomach) pain, bloody diarrhea, vomiting, fever, and/or chills occur with Tolak Cream use.
- Inform patient that these symptoms could be manifestations of a deficiency in the enzyme dihydropyrimidine dehydrogenase (DPD) [see Warnings and Precautions (5.6)] .
- SPL UNCLASSIFIED SECTION
-
PATIENT INFORMATION
Tolak (tol lak)
(fluorouracil)
Cream, 4%
Important: Tolak Cream is for use on skin only (topical). Do not get or apply Tolak Cream on your eyelids or in your eyes, nose, mouth, or in the vagina, because it may cause irritation and swelling in these areas.
What is Tolak Cream?
Tolak Cream is a prescription medicine used to treat skin lesions called actinic keratosis on the face, ears, or scalp.
It is not known if Tolak Cream is safe and effective for use on other areas of the body or to treat problems other than actinic keratosis.
It is not known if Tolak Cream is safe and effective in children.
Who should not use Tolak Cream?
Do not use Tolak Cream if:
- you are pregnant or may become pregnant. Tolak Cream may harm your unborn child. Stop using Tolak Cream and tell your doctor right away if you become pregnant while using Tolak Cream.
- your body does not make enough of (you are deficient in) the enzyme called dihydropyrimidine dehydrogenase (DPD). If you do not have enough of this enzyme, you may get serious side effects if you use Tolak Cream. Symptoms of serious side effects include: stomach-area abdominal pain, bloody diarrhea, vomiting, fever, and chills.
What should I tell my doctor before using Tolak Cream?
Before using Tolak Cream, tell your doctor about all your medical conditions, including if you:
- are allergic to any of the ingredients in Tolak Cream. Tolak Cream contains peanut oil. See the end of this leaflet for a list of all of the ingredients in Tolak Cream.
- are pregnant or plan to become pregnant.
See "Who should not use Tolak Cream?"
- Females who are able to become pregnant should use an effective method of birth control during treatment with Tolak Cream and for one month after the last dose of Tolak Cream. Talk to your doctor about birth control methods that may be right for you during treatment with Tolak Cream.
- are breastfeeding or plan to breastfeed. It is not known if Tolak Cream passes into your breast milk. You and your doctor should decide if you will use Tolak Cream or breastfeed.
How should I use Tolak Cream?
- Use Tolak Cream exactly as prescribed by your doctor.
- Apply Tolak Cream 1 time each day, to the areas of your skin to be treated, for 4 weeks. Apply Tolak Cream as follows:
- Gently wash, rinse, and pat dry the skin areas to be treated.
- Apply a thin film of the Tolak Cream to the areas to be treated.
- Gently massage Tolak Cream evenly into your skin.
- Avoid contact with other areas of your body, and transfer of Tolak Cream from your body to other people.
- Wash your hands well after you apply Tolak Cream.
What should I avoid while using Tolak Cream?
- Do not cover the treated areas with an airtight dressing. Avoid sunlight. Tolak Cream can make your skin sensitive to the sun. You could get severe sunburn. Limit your time in the sun during treatment with Tolak Cream. Talk to your doctor if you get sunburn.
What are the possible side effects of Tolak Cream?
Tolak Cream can cause serious side effects:
- Skin reactions including possible allergic reactions. Most people using Tolak Cream get skin reactions in the treated areas. You may get skin reactions such as:
- redness
- dryness or scaling
- crusting
- itching
- stinging or burning
- swelling
- skin loss (erosion)
-
Eye problems. Eye problems have happened with the use of medicines that contain fluorouracil that are applied to the skin. To help prevent getting Tolak Cream in your eyes, or transferring Tolak Cream from another part of your body to your eyes or to another person's eyes:
- avoid applying Tolak Cream near or around your eyes
- wash your hands well after you apply Tolak Cream
If you accidentally get Tolak Cream in your eyes, or if Tolak Cream is accidentally transferred to another person's eyes, flush eyes with large amounts of water and get medical help as soon as possible.
- Tolak Cream can cause serious side effects in people who do not have enough of the enzyme dihydropyrimidine dehydrogenase (DPD). See "Who should not use Tolak Cream?" and "What should I tell my doctor before using Tolak Cream?" Stop using Tolak Cream and call your doctor right away if you get any of the following symptoms during treatment with Tolak Cream:
- stomach-area (abdominal) pain
- bloody diarrhea
- vomiting
- fever
o chills
Tell your doctor if you get any of these skin reactions and they are severe or do not go away.
These are not all the possible side effects of Tolak Cream. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store Tolak Cream?
- Store Tolak Cream between 68°F to 77°F (20°C to 25°C).
- Do not freeze Tolak Cream.
- Do not use Tolak Cream after the expiration date printed on the tube.
Keep Tolak Cream and all medicines out of the reach of children.
General information about the safe and effective use of Tolak Cream
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Tolak Cream for a condition for which it was not prescribed. Do not give Tolak Cream to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your pharmacist or doctor for information about Tolak Cream that is written for health professionals.
What are the ingredients in Tolak Cream?
Active ingredient: fluorouracil
Inactive ingredients: arlacel-165, butylated hydroxytoluene, cetyl alcohol, anhydrous citric acid, glycerin, isopropyl myristate, methyl gluceth-10, methylparaben, propylparaben, purified water, peanut oil, sodium hydroxide, stearic acid, and stearyl alcohol.
Distributed by: Pierre Fabre Pharmaceuticals, Inc., Parsippany, NJ 07054 USA
For more information, go to www.TOLAK.com or call 1-855-MYTOLAK (698-6525).
This Patient Information has been approved by the U.S. Food and Drug Administration.
Issued: 03/2017
-
PRINCIPAL DISPLAY PANEL - 40 g Tube Carton
NDC: 64370-421-40
Tolak®
(fluorouracil) Cream 4%For Topical Use Only. Not for Ophthalmic, Oral or Intravaginal Use
Rx only
Contains Peanut Oil
40 g
Topical Cream
-
INGREDIENTS AND APPEARANCE
TOLAK
fluorouracil creamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 64370-421 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FLUOROURACIL (UNII: U3P01618RT) (FLUOROURACIL - UNII:U3P01618RT) FLUOROURACIL 40 mg in 1 g Inactive Ingredients Ingredient Name Strength GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) POLYOXYL 100 STEARATE (UNII: YD01N1999R) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) CETYL ALCOHOL (UNII: 936JST6JCN) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) GLYCERIN (UNII: PDC6A3C0OX) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) METHYL GLUCETH-10 (UNII: N0MWT4C7WH) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) PEANUT OIL (UNII: 5TL50QU0W4) SODIUM HYDROXIDE (UNII: 55X04QC32I) STEARIC ACID (UNII: 4ELV7Z65AP) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 64370-421-40 1 in 1 CARTON 03/15/2017 1 40 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC: 64370-421-83 8 in 1 CARTON 02/19/2019 2 1 in 1 CARTON 2 NDC: 64370-421-03 3.5 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022259 03/15/2017 12/31/2020 Labeler - Pierre Fabre Pharmaceuticals, Inc. (968997101) Registrant - Pierre Fabre Pharmaceuticals, Inc. (968997101)
Trademark Results [TOLAK]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 TOLAK 85529383 4818184 Live/Registered |
Hill Dermaceuticals, Inc. 2012-01-31 |
 TOLAK 77464979 not registered Dead/Abandoned |
Hill Dermaceuticals, Inc. 2008-05-02 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.