Naturium Salicylic Acid Serum 2%
Salicylic Acid by
Drug Labeling and Warnings
Salicylic Acid by is a Otc medication manufactured, distributed, or labeled by The Center Brands, LLC, DIMENSIONAL MERCHANDISING INC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
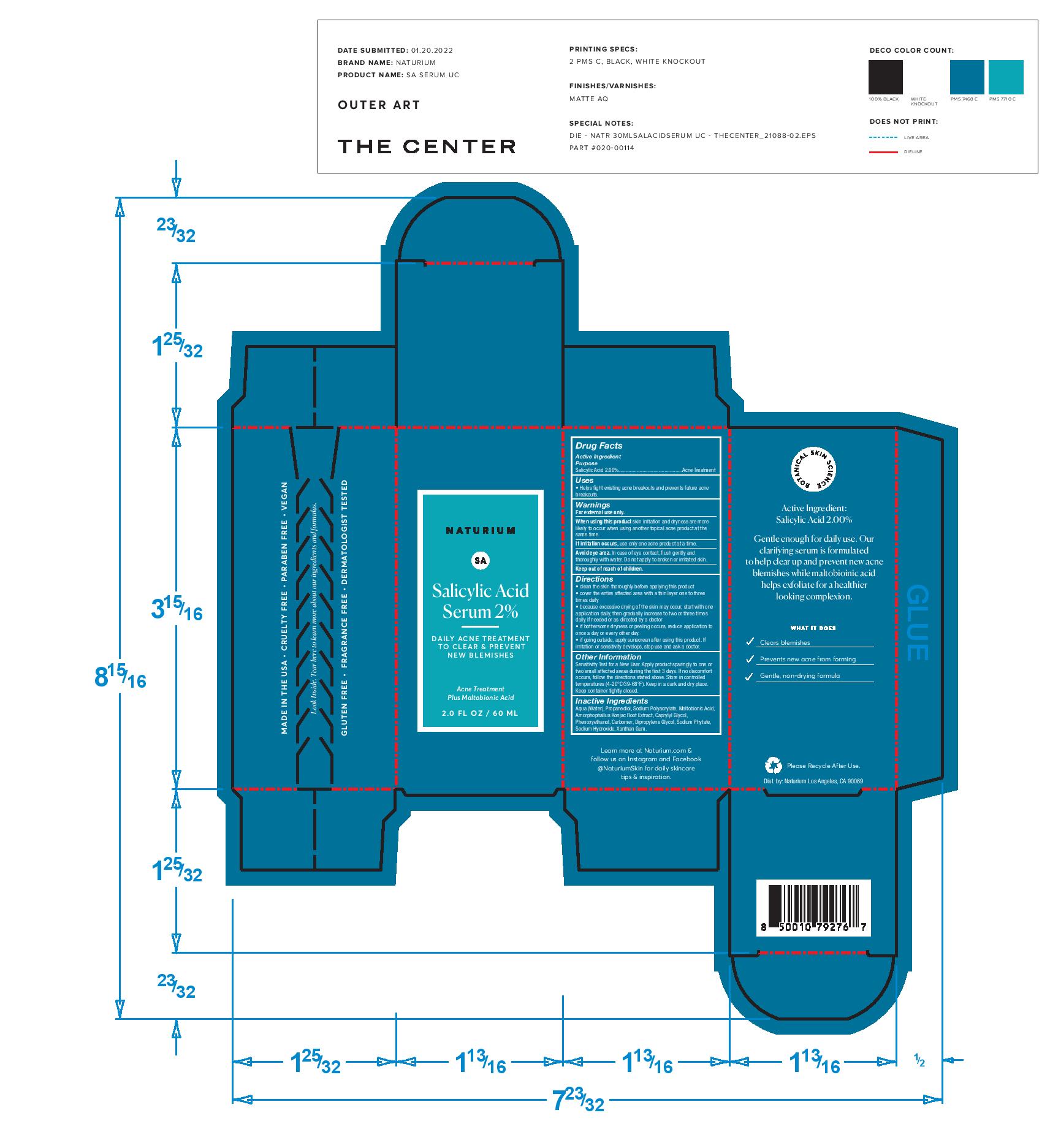
SALICYLIC ACID- salicylic acid serum 2% cream
The Center Brands, LLC
----------
Naturium Salicylic Acid Serum 2%
Directions
clean the skin thoroughly before applying this product
cover the entire affected area with a thin layer one to three
times daily
because excessive drying of the skin may occur, start with one
application daily, then gradually increase to two or three times
daily if needed or as directed by a doctor
if bothersome dryness or peeling occurs, reduce application to
once a day or every other day.
if going outside, apply sunscreen after using this product. If
irritation or sensitivity develops, stop use and ask a doctor.
Directions
clean the skin thoroughly before applying this product
cover the entire affected area with a thin layer one to three
times daily
because excessive drying of the skin may occur, start with one
application daily, then gradually increase to two or three times
daily if needed or as directed by a doctor
if bothersome dryness or peeling occurs, reduce application to
once a day or every other day.
if going outside, apply sunscreen after using this product. If
irritation or sensitivity develops, stop use and ask a doctor.
Warnings
For external use only.
When using this product skin irritation and dryness are more
likely to occur when using another topical acne product at the
same time.
If irritation occurs, use only one acne product at a time.
Avoid eye area. In case of eye contact, flush gently and
thoroughly with water. Do not apply to broken or irritated skin.
Keep out of reach of children.
If irritation occurs, use only one acne product at a time.
Avoid eye area. In case of eye contact, flush gently and
thoroughly with water. Do not apply to broken or irritated skin.
Other Information
Sensitivity Test for a New User. Apply product sparingly to one or
two small affected areas during the first 3 days. If no discomfort
occurs, follow the directions stated above. Store in controlled
temperatures (4-20°C/39-68°F). Keep in a dark and dry place.
Keep container tightly closed.
| SALICYLIC ACID
salicylic acid serum 2% cream |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - The Center Brands, LLC (076228814) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| DIMENSIONAL MERCHANDISING INC | 076693183 | manufacture(82800-003) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.