KT RECOVER MAGNESIUM by KT Health LLC / Inspec Solutions, LLC
KT RECOVER MAGNESIUM by
Drug Labeling and Warnings
KT RECOVER MAGNESIUM by is a Otc medication manufactured, distributed, or labeled by KT Health LLC, Inspec Solutions, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
KT RECOVER MAGNESIUM- menthol cream
KT Health LLC
----------
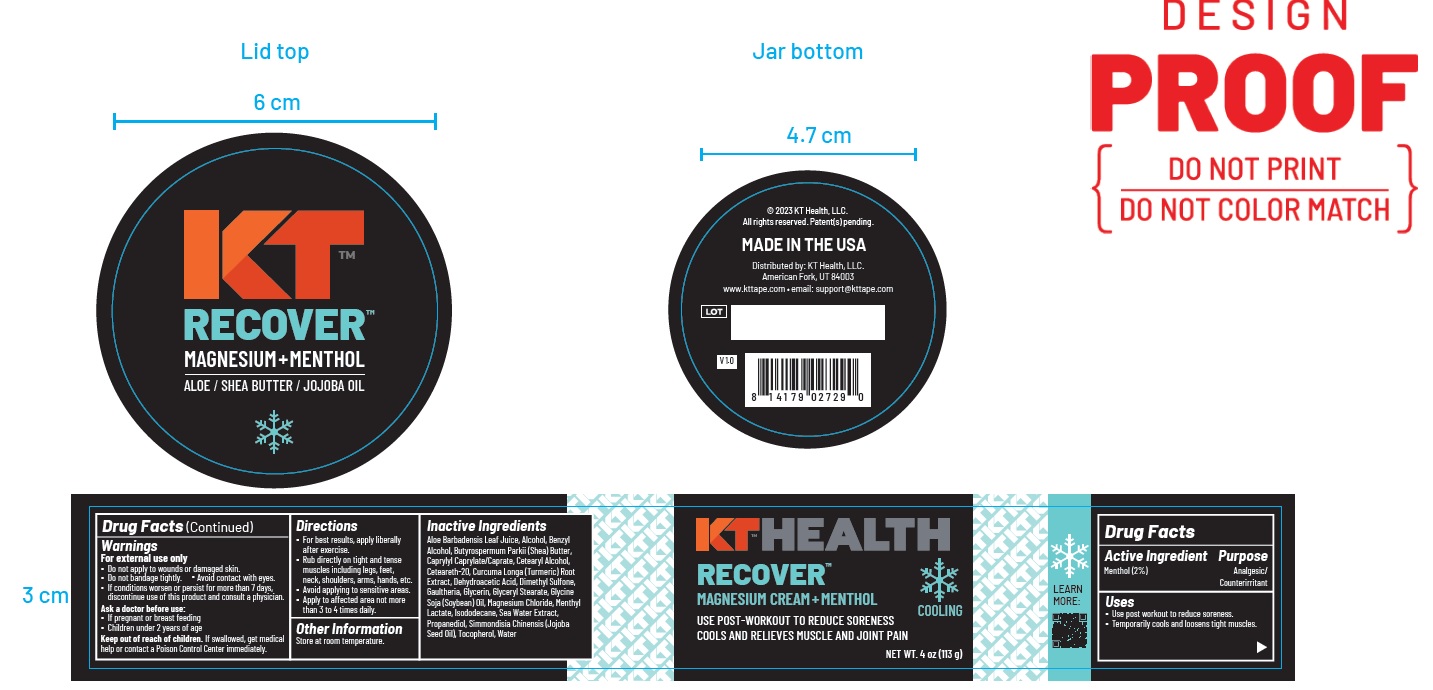
Warnings
For external use only
- Do not apply to wounds or damaged skin.
- Do not bandage tightly.
- Avoid contact with eyes.
- If condition worsens, or persist for more than 7 days, discontinue use of this product and consult a physician.
- Ask a doctor before use:
- If pregnant or breast feeding
- Children under 2 years of age
Keep out of reach of children.If swallowed, get medical
help or contact a Poison Control Center immediately.
Directions
- For best results, apply liberally after exercise.
- Rub directly on tight and tense muscles including legs, feet,neck, shoulders, arms, hands, etc.
- Avoid applying to sensitive areas.
- Apply to affected area not more than 3 to 4 times daily.
Aloe Barbadensis Leaf Juice, Alcohol, Benzyl Alcohol, Butyrospermum Parkii (Shea) Butter, Caprylyl Caprylate/Caprate, Cetearyl Alcohol,
Ceteareth-20, Curcuma Longa (Turmeric) Root Extract, Dehydroacetic Acid, Dimethyl Sulfone,Gaultheria, Glycerin, Glyceryl Stearate, Glycine
Soja (Soybean) Oil, Magnesium Chloride, Menthyl Lactate, Isododecane, Sea Water Extract, Propanediol, Simmondisia Chinensis (Jojoba Seed Oil), Tocopherol, Water
| KT RECOVER MAGNESIUM
menthol cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - KT Health LLC (807008037) |
| Registrant - Inspec Solutions, LLC (081030372) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Inspec Solutions, LLC | 081030372 | manufacture(73044-104) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.