INTOMEDI FIRST AMPOULE EGF- adenosine liquid
INTOMEDI FIRST AMPOULE EGF by
Drug Labeling and Warnings
INTOMEDI FIRST AMPOULE EGF by is a Otc medication manufactured, distributed, or labeled by Janytree Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
INACTIVE INGREDIENTS
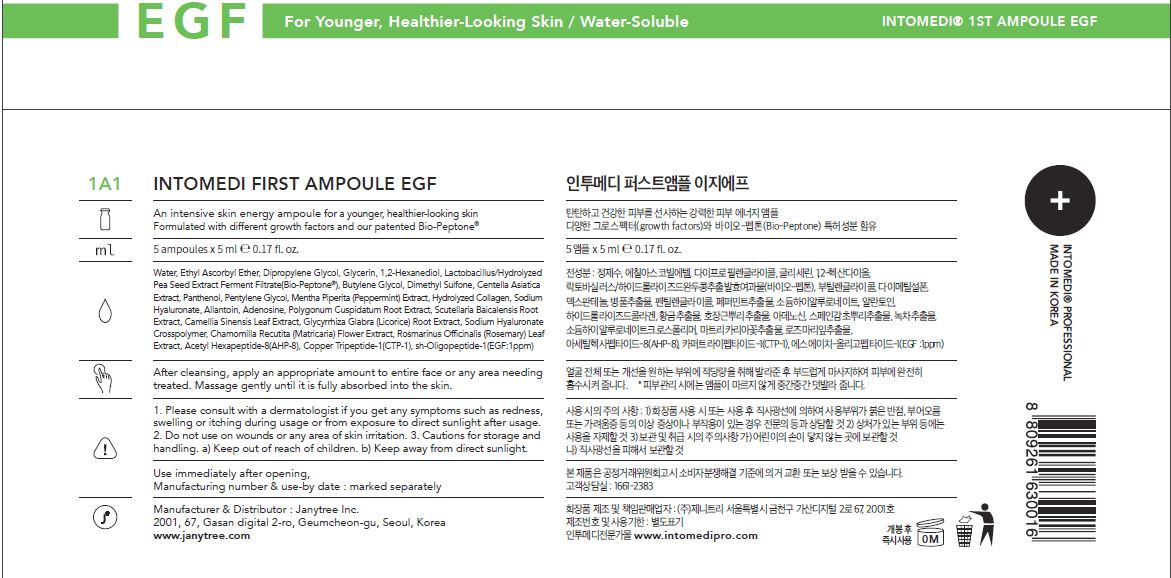
Water, Ethyl Ascorbyl Ether, Dipropylene Glycol, Glycerin, 1,2-Hexanediol, Lactobacillus/Hydrolyzed
Pea Seed Extract Ferment Filtrate(Bio-Peptone®), Butylene Glycol, Dimethyl Sulfone, Centella Asiatica
Extract, Panthenol, Pentylene Glycol, Mentha Piperita (Peppermint) Extract, Hydrolyzed Collagen, Sodium
Hyaluronate, Allantoin, Polygonum Cuspidatum Root Extract, Scutellaria Baicalensis Root
Extract, Camellia Sinensis Leaf Extract, Glycyrrhiza Glabra (Licorice) Root Extract, Sodium Hyaluronate
Crosspolymer, Chamomilla Recutita (Matricaria) Flower Extract, Rosmarinus Officinalis (Rosemary) Leaf
Extract, Acetyl Hexapeptide-8(AHP-8), Copper Tripeptide-1(CTP-1), sh-Oligopeptide-1(EGF:1ppm) - ACTIVE INGREDIENTS
- PURPOSE
-
WARNINGS
For external use only When using this product
■ If following abnormal symptoms occurs after using the product, stop using the product and consult with a skin specialist. Symptoms : Red specks, swelling, itching
■ Do not use on the skin parts affected by wound, eczema, or dermatitis.
Keep out of reach of children.
■ If swallowed, get a medical help or contact a person in control center immediately.
■ Avoid contact with eyes. - KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
INTOMEDI FIRST AMPOULE EGF
adenosine liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 82879-0003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ADENOSINE (UNII: K72T3FS567) (ADENOSINE - UNII:K72T3FS567) ADENOSINE 0.04 g in 100 mL Inactive Ingredients Ingredient Name Strength BUTYLENE GLYCOL (UNII: 3XUS85K0RA) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 82879-0003-2 5 in 1 PACKAGE 07/13/2022 1 NDC: 82879-0003-1 5 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 07/13/2022 Labeler - Janytree Inc. (688403840) Registrant - Janytree Inc. (688403840) Establishment Name Address ID/FEI Business Operations Janytree Inc. 688403840 manufacture(82879-0003)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.