ZYCUBO- copper histidinate injection, powder, lyophilized, for solution
Zycubo by
Drug Labeling and Warnings
Zycubo by is a Prescription medication manufactured, distributed, or labeled by Sentynl Therapeutics, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ZYCUBO safely and effectively. See full prescribing information for ZYCUBO.
ZYCUBO® (copper histidinate) for injection, for subcutaneous use
Initial U.S. Approval: 2026INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
- Before initiating ZYCUBO, obtain baseline serum copper and ceruloplasmin levels, serum electrolytes, kidney and liver function, and complete blood count. (2.1)
- The recommended dosage of ZYCUBO in pediatric patients:
- Monitor serum copper and ceruloplasmin levels, serum electrolytes, kidney and liver function, and complete blood count (CBC). (2.3)
- Reconstitute ZYCUBO and administer subcutaneously. (2.4, 2.6)
- See Full Prescribing Information for additional preparation, storage, and administration instructions. (2.4, 2.5, 2.6)
DOSAGE FORMS AND STRENGTHS
For Injection: 2.9 mg of copper histidinate (equivalent to 0.5 mg elemental copper) as a lyophilized powder or cake in a single-dose vial for reconstitution. (3)
CONTRAINDICATIONS
- None (4)
WARNINGS AND PRECAUTIONS
Copper Accumulation and Risk of Toxicity: Exogenous administration of copper with ZYCUBO may lead to further copper accumulation and has the potential to result in drug-induced kidney injury, liver dysfunction, and hematological abnormalities. Monitor patients during ZYCUBO treatment. Adjust dosage if necessary. (2.2, 5.1, 6.1)
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥7%) were pneumonia, viral infection, respiratory failure, seizure, bacterial infection, hemorrhage, hypotension, vomiting, tachycardia, pyrexia, volume depletion, fracture, dyspnea, transaminases elevation, diarrhea, fungal infection, anemia, and local administration reaction. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Sentynl Therapeutics, Inc. at 1-888-507-5206 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 1/2026
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Testing Before Initiating ZYCUBO
2.2 Recommended Dosage and Administration
2.3 Dosage and Administration Modifications and Monitoring
2.4 Preparation Instructions
2.5 Storage of Reconstituted Solution
2.6 Administration Instructions
2.7 Missed Dose
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Copper Accumulation and Risk of Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Testing Before Initiating ZYCUBO
Before initiating ZYCUBO, obtain baseline serum copper and ceruloplasmin levels, serum electrolytes, kidney and liver function, and complete blood count (CBC) [see Warnings and Precautions (5.1)].
2.2 Recommended Dosage and Administration
The recommended dosage of ZYCUBO in pediatric patients:
- Less than 1 year of age is 1.45 mg administered subcutaneously twice daily (8-12 hours between injections).
- 1 year of age to less than 17 years of age is 1.45 mg administered subcutaneously once daily.
2.3 Dosage and Administration Modifications and Monitoring
Monitor serum copper and ceruloplasmin levels, serum electrolytes, kidney and liver function, and complete blood count (CBC) every 6 weeks for the first 6 months, then every 3 months for 18 months, and then every 6 months thereafter during ZYCUBO treatment. If laboratory abnormalities are detected, consider reducing the frequency of ZYCUBO administration or temporarily withholding or permanently discontinuing ZYCUBO. Return to increased frequency of laboratory evaluation when resuming a dosage as clinically indicated [see Warnings and Precautions (5.1)].
2.4 Preparation Instructions
Preparation
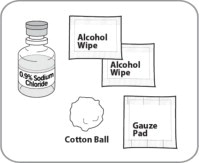
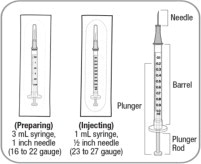
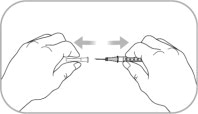
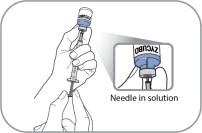
- Use aseptic technique during preparation. Reconstitute ZYCUBO using a sterile disposable 3 mL syringe and 1 inch needle (between 16 to 22 gauge) (see Instructions for Use).
- Remove 1 ZYCUBO vial from the refrigerator and set aside for approximately 30 minutes to allow the vial to come to room temperature [20°C to 25°C (68°F to 77°F)] before use.
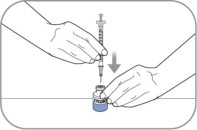
- Reconstitute ZYCUBO by tilting the vial and slowly injecting 1 mL of 0.9% Sodium Chloride Injection, USP down the inside wall of the vial.
- Gently swirl the vial continuously until the powder is completely dissolved. Do not shake the vial. Each vial will yield a concentration of 2.9 mg/mL.
- Visually inspect the reconstituted solution in the vial for particulate matter and discoloration. The solution should be blue. Discard if particles are present or the solution is discolored (not blue) or cloudy.
- Do not mix with other medications.
2.5 Storage of Reconstituted Solution
If the reconstituted ZYCUBO vial is not used immediately, store the vial refrigerated at 2°C to 8°C (36° to 46°F) for up to 24 hours or at controlled room temperature at 20°C to 25°C (68°F to 77°F) for up to 4 hours.
Discard the reconstituted ZYCUBO vial if not used within 24 hours of refrigeration or within 4 hours at room temperature.
2.6 Administration Instructions
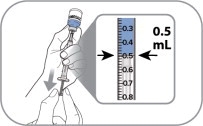
A caregiver may administer ZYCUBO to patients after proper training in subcutaneous injection technique if a healthcare provider determines that it is appropriate (see Instructions for Use). Administer ZYCUBO using a sterile disposable 1 mL syringe and 1/2 inch injection needle (between 23 to 27 gauge).
Slowly withdraw 0.5 mL of reconstituted ZYCUBO solution from the vial and inject subcutaneously.
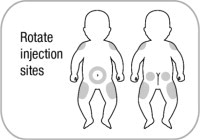
Administer ZYCUBO by subcutaneous injection at separate sites in the abdominal area (2 inches from the navel), buttocks, and the outer lateral aspect of the upper arm or thigh. Rotate injection sites with each injection to reduce the risk of lipodystrophy. Do not give injections into areas where the skin is scarred, tender, bruised, red, or hard.
Discard unused portion after each single use. Do not administer more than one dose from the vial.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Copper Accumulation and Risk of Toxicity
Impaired copper transport in patients with Menkes disease can lead to copper accumulation and organ impairment in the kidneys, liver, and hematopoietic system. Treatment with ZYCUBO may lead to further copper accumulation and related toxicity, especially in the first two years of life given renal and hepatic immaturity.
Obtain baseline serum copper and ceruloplasmin levels, serum electrolytes, kidney and liver function, and complete blood count (CBC). After initiating ZYCUBO, monitor laboratory values every 6 weeks for the first 6 months, then every 3 months for 18 months, and then every 6 months thereafter during ZYCUBO treatment. If laboratory abnormalities are detected, consider reducing the frequency of ZYCUBO administration or temporarily withholding or permanently discontinuing ZYCUBO. Return to increased frequency of laboratory monitoring when resuming a dosage as clinically indicated.
Drug-Induced Kidney Injury
Copper accumulation with ZYCUBO use has the potential to result in renal tubular toxicity in patients with Menkes disease. Routinely monitor patients starting or re-starting ZYCUBO for signs and symptoms of renal tubular toxicity. New-onset or worsening non-anion gap metabolic acidosis may be a sign of drug-related renal tubular acidosis. Increased urinary beta-2 microglobulin and/or new-onset hypophosphatemia, hyponatremia, or hypokalemia may be signs of drug-related proximal renal tubular toxicity. Provide supportive care with electrolyte repletion and supplementation as clinically indicated.
Copper accumulation with ZYCUBO use has the potential to result in glomerular injury, leading to decreased kidney function or new-onset proteinuria.
Liver Dysfunction
Copper accumulation with ZYCUBO can result in liver dysfunction. Elevations of liver transaminases have been reported in patients taking ZYCUBO for Menkes disease [see Adverse Reactions (6.1)]. Single cell necrosis, inflammation, and fibrosis, along with increased liver transaminases and bilirubin were observed in studies conducted over 13-weeks in juvenile rats with normal baseline copper levels [see Use in Specific Populations (8.4)].
Hematological Abnormalities
Copper accumulation with ZYCUBO can result in spleen and bone marrow dysfunction as well as interference with iron metabolism. Anemia has been reported in patients taking ZYCUBO for Menkes disease [see Adverse Reactions (6.1)]. Increased cellularity and pigmented macrophages in the spleen and increased hematological values were observed in studies conducted over 13-weeks in normal juvenile rats [see Use in Specific Populations (8.4)].
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Copper Accumulation and Risk of Toxicity: Drug-Induced Kidney Injury, Liver Dysfunction, Hematological Abnormalities [see Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The pooled safety analysis from 2 open-label, single-arm clinical trials included a total of 129 ZYCUBO-treated patients with an age range from 0 to 48 months. Patients less than 1 year of age received ZYCUBO 1.45 mg twice daily, and patients 1 year of age and older received ZYCUBO 1.45 mg once daily. The median exposure duration was 24 months (range: 1 to 39 months) [see Clinical Studies (14)].
Serious Adverse Reactions
Serious adverse reactions reported in ≥5% of ZYCUBO-treated pediatric patients with Menkes disease were pneumonia, dehydration, seizure, respiratory distress, respiratory syncytial virus infection, cardiopulmonary failure, upper respiratory tract infection, respiratory failure, and vomiting.
Common Adverse Reactions
Table 1 lists the most common adverse reactions that occurred in ≥7% of patients in the pooled safety analysis during an observation period ranging from 1 to 39 months.
Table 1. Adverse Reactions Occurring in ≥7% Patients with Menkes Disease (Trial 1 and Trial 2) 1Respiratory failure consists of multiple similar terms including cardiopulmonary failure.
2Bacterial infection consists of multiple similar terms including renal and urinary tract infection.
Adverse Reactions Menkes Disease (N = 129)
N (%)Pneumonia 38 (30) Viral infection 35 (27) Respiratory failure1 30 (23) Cardiopulmonary failure 11 (9) Seizure 29 (23) Bacterial infection 26 (20) Renal and urinary tract infection2 12 (9) Hemorrhage 23 (18) Hypotension 20 (16) Vomiting 19 (15) Tachycardia 16 (12) Pyrexia 16 (12) Volume depletion 16 (12) Fracture 16 (12) Dyspnea 16 (12) Transaminases elevation 13 (10) Diarrhea 13 (10) Fungal infection 12 (9) Anemia 11 (9) Local administration reaction 9 (7) -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on ZYCUBO use during pregnancy to evaluate for a drug-associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes. Animal reproduction studies have not been conducted with ZYCUBO.
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defects, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2%-4% and 15%-20%, respectively.
8.2 Lactation
Risk Summary
There are no data on the presence of copper histidinate and its metabolites in either human or animal milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for ZYCUBO and any potential adverse effects on the breastfed infant from ZYCUBO or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of ZYCUBO for the treatment of Menkes disease have been established in pediatric patients, and the information on this use is discussed throughout the labeling.
Juvenile Animal Toxicity Data
Juvenile rats with normal baseline copper levels were administered copper histidinate from postnatal day (PND) 10 (the equivalent of a human newborn) to PND 100 (the equivalent of a human adult) subcutaneously twice daily for 13 weeks at 1, 2, and 5 mg/kg. Histopathological findings were observed in the kidney (tubular necrosis, eosinophilic globules), liver (single cell necrosis, inflammation, fibrosis), and spleen (increased cellularity and pigmented macrophages), in addition to increased liver transaminases (ALT, AST) and bilirubin, and decreased red blood cells, hemoglobin and hematocrit at 5 mg/kg (10-fold the human plasma concentration at the recommended dose of ZYCUBO (based on Cmax)). Changes in liver (necrosis, inflammation, ALT, AST) and kidney (eosinophilic globules) were also noted as low as 1 mg/kg (equivalent to human plasma concentration at the recommended dose of ZYCUBO (based on Cmax)). A no-observed-adverse-effect-level (NOAEL) in juvenile rats could not be determined. Proportional increases in copper and ceruloplasmin levels occurred with increasing dose levels.
-
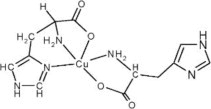
11 DESCRIPTION
ZYCUBO (copper histidinate) for injection is a copper replacement product. The chemical name is copper, (L-histidinato-ϰN,ϰN3,ϰO)(L-histidinato-ϰN,ϰO)-, (SP-5-14-C)-. The molecular formula is C12H16CuN6O4, and the molecular weight is 371.84 g/mol. Copper histidinate is soluble in water.
The chemical structure is:
ZYCUBO is a sterile, preservative-free, blue lyophilized powder or cake for subcutaneous injection after reconstitution with 1 mL sterile 0.9% Sodium Chloride Injection, USP. Each single-dose vial contains 2.9 mg of copper histidinate (equivalent to 0.5 mg elemental copper). The resultant solution has a concentration of 2.9 mg/mL and a pH of 7.4.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Menkes disease is an X-linked recessive disorder caused by pathogenic variants in the copper transport ATPase encoded by ATP7A. Patients with Menkes disease have impaired absorption of copper from their diet, impaired transport of copper across the blood-brain barrier, and dysregulation of many copper-dependent enzymes. ZYCUBO is a bioavailable copper replacement therapy that is administered as a subcutaneous injection to bypass the impaired gastrointestinal absorption observed in patients with Menkes disease.
12.2 Pharmacodynamics
Patients with Menkes disease have low serum concentrations of copper and ceruloplasmin. Treatment with ZYCUBO increases serum copper and ceruloplasmin concentrations in patients with Menkes disease.
The exposure-response relationship and time course of pharmacodynamic response for the safety and effectiveness of ZYCUBO have not been fully characterized.
12.3 Pharmacokinetics
The geometric mean (CV%) maximum serum concentration (Cmax) of copper histidinate was 67 (36%) ng/mL, the geometric mean (CV%) area under the concentration-time curve from time 0 to 24 hours (AUC0-24hr) was 186 (21%) nghr/mL, and the geometric mean (CV%) area under the concentration-time curve from time 0 to infinity (AUC0-inf) was 296 (15%) nghr/mL following a single subcutaneous dose of 3 mg copper histidinate (approximately twice the approved recommended dose for Menkes disease patients 1 year old or older) in healthy adult subjects.
At the recommended dosage, the mean (SD) serum copper concentration increased from a baseline concentration of 30 (25) mcg/dL to 114 (38) mcg/dL at 12 months, and gradually decreased over the 36-month treatment period, with a mean (SD) serum copper concentration of 63 (31) mcg/dL at 36 months. The mean (SD) serum ceruloplasmin concentration was 12 (12) mg/dL at baseline, 33 (11) mg/dL at 12 months, and 20 (8) mg/dL at 36 months [see Clinical Studies (14)].
Absorption
The absolute bioavailability of copper histidinate following subcutaneous injection has not been determined. The median time to reach maximum serum concentrations of copper histidinate (Tmax) was 0.75 hours following a single subcutaneous dose of 3 mg copper histidinate (approximately twice the approved recommended dose for Menkes disease patients 1 year old or older) in healthy adult subjects.
Distribution
The mean (SD) apparent volume of distribution (Vz/F) of copper histidinate during the terminal elimination phase was 1034 (588) L following a single subcutaneous dose of 3 mg copper histidinate (approximately twice the approved recommended dose for Menkes disease patients 1 year old or older) in healthy adult subjects.
Serum copper histidinate concentration-time profiles exhibited an initial decrease followed by a terminal elimination phase, consistent with the release of copper from the copper histidinate complex to the carrier proteins ceruloplasmin and albumin, followed by incorporation of copper histidinate and histidine into the endogenous pools of copper histidinate and histidine, respectively.
No binding of copper histidinate to human plasma proteins in vitro has been observed.
Elimination
The mean (SD) serum clearance (CL/F) of copper histidinate was 10.3 (1.6) L/hr, and the mean terminal half-life was 75 hours following a single subcutaneous dose of 3 mg copper histidinate (approximately twice the approved recommended dose for Menkes disease patients 1 year old or older) in healthy adult subjects.
Drug Interaction Studies
In vitro Studies: Copper histidinate is a weak inhibitor of CYP1A2, CYP2B6, CYP2C9, CYP2C19, and CYP2D6 (IC50 >100 μM). Copper histidinate is not an inducer of CYP1A2, CYP2B6, or CYP3A.
Transporter Systems: Copper histidinate is not a substrate of BCRP, P-gp, MATE1, MATE2-K, OAT1, OAT3, OATP1B1, OAT1B3, or OCT2. Copper histidinate is an inhibitor of BSEP and MATE1. Copper histidinate is a weak inhibitor of BCRP, MATE2K, OCT1, and OCT2. Copper histidinate is not an inhibitor of P-gp, OAT1, OAT3, OATP1B1, or OATP1B3.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Animal studies to evaluate the carcinogenic potential of copper histidinate have not been conducted with ZYCUBO.
-
14 CLINICAL STUDIES
The efficacy of ZYCUBO was evaluated in pediatric patients with Menkes disease (age at treatment initiation ranges 0.1 to 31.4 months) receiving 3 years of copper histidinate treatment in two open-label, single-arm clinical trials (Trial 1, NCT00001262 and Trial 2, NCT00811785). Data from ZYCUBO-treated patients in these two trials were compared to data from an untreated contemporaneous external control cohort as collected under a protocol amendment of Trial 2. In both trials, pediatric patients:
- Less than 1 year of age received 1.45 mg of ZYCUBO administered subcutaneously twice daily until 1 year of age.
- Equal to or greater than 1 year of age received 1.45 mg of ZYCUBO subcutaneously once daily for up to 3 years.
Overall survival was evaluated in a subset of the pooled population from Trial 1 and Trial 2, referred to as the pooled efficacy population. This efficacy population included only patients with Menkes disease who carried a severe pathogenic variant of the ATP7A gene (duplication/deletion, nonsense, or a canonical splice junction variant) and were born after 1999. There were 83 pediatric patients (66 ZYCUBO; 17 external control) in this pooled efficacy population: 21 patients (21 ZYCUBO) from Trial 1 and 62 patients (45 ZYCUBO; 17 external control) from Trial 2.
Patients in the pooled efficacy population were assigned to 1 of 4 cohorts as described in Table 2.
Table 2. Patient Cohorts in the Pooled Efficacy Population All values are in median (range)
1 Within 4 weeks of birth or within 4 weeks of birth corrected for prematurity (i.e., < 40 weeks' gestation)
2 After 4 weeks of birth or after 4 weeks of birth corrected for prematurity (i.e., < 40 weeks' gestation)
Treated Cohorts Untreated Cohorts ZYCUBO- Early Treatment
(ZYCUBO-ET)
n=31ZYCUBO- Late Treatment
(ZYCUBO-LT)
n=35External Control-Early Treatment
(EC-ET)
n=17External Control-Late Treatment
(EC-LT)
n=16Eligibility Started ZYCUBO treatment within 4 weeks of birth [1] Started ZYCUBO treatment after 4 weeks of birth [2] - No prior ZYCUBO or copper treatment
- Asymptomatic for significant neurological signs and symptoms approximately 4 weeks after birth
- Survived at least 4 weeks after diagnosis
- Subset of the EC-ET cohort
- Diagnosed with Menkes disease after 4 weeks of birth
- Survived at least 2 weeks after diagnosis
Age at diagnosis (months) 0.1 (-4.5 – 1.9) 4.8 (0.4 – 29.4) 4.7 (2.1 – 22.2) 5.6 (2.1 – 22.2) Age at treatment initiation (months) 0.4 (0.1 – 1.9) 7.1 (1.3 – 31.4) NA NA Treatment duration (months) 34.1 (1.1 – 36) 20 (1.3 – 36) NA NA In the 4 cohorts, 81 patients were male (98%) except for 2 female (2%) patients in ZYCUBO-LT. The pooled efficacy population included patients with the following race and ethnicity: 52 White (63%), 11 Hispanic (13%), 8 Black or African American (10%), 6 Unknown (7%), 4 Other (5%), and 2 Asian or Pacific Islander (2%). The majority of patients in all 4 cohorts were born prematurely: ZYCUBO-ET = 77%, ZYCUBO-LT = 66%, EC-ET = 82%, and EC-LT = 81%.
Efficacy Results
Primary Efficacy Results (Overall Survival) in the ZYCUBO-ET and EC-ET Cohorts
The primary efficacy analysis compared the overall survival in patients in the ZYCUBO-ET and EC-ET cohorts. Patients in the ZYCUBO-ET cohort (patients treated with ZYCUBO) had a significant improvement in overall survival compared to patients in the EC-ET cohort, with a 78% reduction in the risk of death (Table 3 and Figure 1).
In the ZYCUBO-ET cohort, 15 (48%) patients survived >6 years, including 7 (23%) patients who survived >12 years. In the EC-ET cohort, no patients survived >6 years.
Table 3. Primary Efficacy Results: Overall Survival in ZYCUBO Early Treatment and External Control Early Treatment Cohorts with Menkes Disease CI=Confidence Interval; NE=Not estimable
Note: If death dates were unknown, patients were censored at the last known date alive.
ZYCUBO-Early Treatment
(n=31)External Control-Early Treatment
(n=17)Number (%) of Patients Alive 16 (52%) 2 (12%) Median survival time (months) (95% CI) 177.1 (33, NE) 17.6 (11.5, 28.6) Hazard Ratio (95% CI) 0.22 (0.10, 0.49) Figure 1. Kaplan-Meier Overall Survival Curve for the ZYCUBO Early Treatment and External Control Early Treatment Cohorts with Menkes Disease
CuHis=copper histidinate; ET=early treatment; EC=external control
Secondary Efficacy Results (Overall Survival) in the ZYCUBO-LT and EC-LT Cohorts
The secondary efficacy analysis compared the overall survival in patients in the ZYCUBO-LT cohort with patients in the EC-LT cohort. Patients in the ZYCUBO-LT cohort (patients treated with ZYCUBO) had a significant improvement in overall survival compared to patients in the EC-LT cohort, with a 73% reduction in the risk of death (Table 4 and Figure 2).
Table 4. Secondary Efficacy Results: Overall Survival in ZYCUBO Late Treatment and External Control Late Treatment Cohorts with Menkes Disease CI=Confidence Interval
Note: If death dates were unknown, patients were censored at the last known date alive.
ZYCUBO Late-Treatment (LT)
(n=35)External Control-Late Treatment (EC-LT)
(n=16)Number of Patients Alive (%) 12 (34%) 2 (12%) Median survival time (months) (95% CI) 62.4 (29.6, 80.7) 20.7 (12.6, 28.6) Hazard Ratio (95% CI) 0.27 (0.12, 0.57) Figure 2. Kaplan-Meier Overall Survival Curve for ZYCUBO Late Treatment and External Control Late Treatment Cohorts with Menkes Disease
CuHis=copper histidinate; LT=late treatment; EC=external control
In the ZYCUBO-LT cohort, 11 (31.4%) patients survived >6 years, including 1 patient (2.9%) who survived >12 years. In the EC-LT cohort, no patients survived >6 years.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
ZYCUBO (copper histidinate) for injection is supplied as a sterile, preservative-free, blue lyophilized powder or cake in a single-dose vial. Each vial contains 2.9 mg of copper histidinate (equivalent to 0.5 mg elemental copper). ZYCUBO is available as:
- One 2.9 mg single-dose vial in a carton: NDC: 42358-329-01
Storage and Handling
Store ZYCUBO vials refrigerated at 2°C to 8°C (36°F to 46°F) in the original carton.
Store ZYCUBO reconstituted solution either refrigerated or at controlled room temperature [see Dosage and Administration (2.5)].
-
17 PATIENT COUNSELING INFORMATION
Advise the patient and/or caregiver to read the FDA-approved patient labeling (Instructions for Use).
Drug-Induced Kidney Injury, Liver Dysfunction, and Hematological Abnormalities
Advise the patient and/or caregiver of the potential for the patient to experience drug-induced kidney injury, liver dysfunction, and hematological abnormalities. [see Warnings and Precautions (5.1)].
Manufactured by:
Zydus Lifesciences Ltd.
Vadodara 391510
IndiaManufactured for:
Sentynl Therapeutics, Inc.
Solana Beach, CA 92075ZYCUBO is a registered trademark of Sentynl Therapeutics, Inc.
-
INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE
ZYCUBO® [zye kyoo boe]
(copper histidinate)
for injection, for subcutaneous use
This Instructions for Use contains information on how to prepare and inject ZYCUBO. Read this Instructions for Use before you prepare and inject a dose of ZYCUBO for the first time and each time you get a ZYCUBO refill. There may be new information. This information does not take the place of talking to your healthcare provider about your child's medical condition or their treatment.
ZYCUBO is for subcutaneous injection only (inject directly under the skin). Always follow the specific instructions given by your healthcare provider.
- If you have questions about preparing or injecting ZYCUBO, call SentynlCares | ZYCUBO Patient Support Services at 1-888-251-2800.
Important information you need to know before preparing and injecting ZYCUBO:
- Your healthcare provider should show you the right way to prepare and inject your child's prescribed dose of ZYCUBO before you do this for the first time.
- Your healthcare provider will prescribe the amount of ZYCUBO needed for each dose for your child. Confirm the amount of ZYCUBO needed at each visit with your child's healthcare provider.
- ZYCUBO comes as a powder or cake in a vial. Each vial of ZYCUBO must be mixed with 0.9% sodium chloride to mix (dissolve) the powder or cake before use.
- Do not mix ZYCUBO with anything other than 0.9% sodium chloride.
- Vials of ZYCUBO are for 1 time use only. Throw the vial away after use, even if there is medicine left in the vial. Do not save for later use. Throw away used vials in your household trash.
- If your child misses a dose of ZYCUBO, inject the dose as soon as possible. Inject the next scheduled dose at least 6 hours after you finish injecting the missed dose.
- Do not expose ZYCUBO to any heat source, such as a microwave or hot water.
- Do not share needles and syringes. See Step 13: “Throw away (dispose of) used needles and syringes.”
Storing ZYCUBO and other supplies:
Vials of ZYCUBO before mixing:
- Store ZYCUBO in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Keep ZYCUBO vials in the original carton until you are ready to use it.
Vials of ZYCUBO after mixing:
- If you do not use the ZYCUBO solution right away after mixing, store the mixed ZYCUBO vial:
- in the refrigerator between 36°F to 46°F (2°C to 8°C) and use within 24 hours. Throw away (discard) the mixed ZYCUBO vial if not used within 24 hours.
- at room temperature between 68°F to 77°F (20°C to 25°C) and use within 4 hours. Throw away (discard) the mixed ZYCUBO vial if not used within 4 hours.
- Write the date and time you mixed ZYCUBO with 0.9% sodium chloride on the carton.
- Do not shake ZYCUBO after it has been mixed.
Other supplies:
- Store other supplies according to the manufacturer instructions (see Step 2: “Gather and check other supplies” for a list of supplies needed).
Keep ZYCUBO and all medicines out of the reach of children.
Preparing and injecting ZYCUBO
-
PRINCIPAL DISPLAY PANEL
Principal Display Panel - 2.9 mg Carton Label
NDC: 42358-329-01
Zycubo ®
(copper histidinate) for injection2.9 mg/vial
For subcutaneous injection
One Single-Dose vial
Discard unused portion after each single use.
Do not administer more than one dose from the vial.Rx only

-
PRINCIPAL DISPLAY PANEL
Principal Display Panel - 2.9 mg Vial Label
NDC: 42358-329-01 Rx only
Zycubo ®
(copper histidinate) for injection2.9 mg/vial
For subcutaneous injection
One Single-Dose vial
Discard unused portion after each single use.
Do not administer more than one dose from the vial.
-
INGREDIENTS AND APPEARANCE
ZYCUBO
copper histidinate injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 42358-329 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength copper histidinate (UNII: 9078K3MO9U) (copper histidinate - UNII:9078K3MO9U) copper histidinate 2.9 mg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42358-329-01 1 in 1 CARTON 01/26/2026 1 1 in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA211241 01/12/2026 Labeler - Sentynl Therapeutics, Inc. (078313706)
Trademark Results [Zycubo]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ZYCUBO 97239527 not registered Live/Pending |
Sentynl Therapeutics, Inc. 2022-01-26 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.