MECLIZINE HYDROCHLORIDE TABLETS, 12.5 mg DRUG FACTS
Meclizine HCl by
Drug Labeling and Warnings
Meclizine HCl by is a Otc medication manufactured, distributed, or labeled by Drug Ocean LLC, Unique Pharmaceutical Laboratories. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
MECLIZINE HCL- meclizine hydrochloride tablet
Drug Ocean LLC
----------
MECLIZINE HYDROCHLORIDE TABLETS, 12.5 mg
DRUG FACTS
Warnings:
Do not take this product, unless directed by a doctor, if you have
- Glaucoma
- A breathing problem such as emphysema or chronic bronchitis
- Trouble urinating due to an enlarged prostate gland.
Do not take this product if you are taking sedatives or tranquilizers, without first consulting your doctor
When using product
- do not exceed recommended dosage
- may cause drowsiness
- alcohol, sedatives, and tranquilizers may increase drowsiness
- avoid alcoholic drinks
- use caution when driving a motor vehicle or operating machinery
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away
Directions
- dosage should be taken one hour before travel starts
| adults and children 12 years and over | take 2 or 4 tablets once daily or as directed by a doctor |
Inactive ingredients
colloidal silicon dioxide, crospovidone, lactose monohydrate, magnesium stearate, microcrystalline cellulose
TAMPER EVIDENT: DO NOT USE IF FOIL SEAL UNDER CAP, PRINTED WITH “SEALED for YOUR PROTECTION” IS BROKEN OR MISSING
Distributed by:
Drug Ocean LLC,
1 Bridge Plaza, North Central Road,
6th Floor, Suite 675,
Fort Lee, NJ 07024
Manufactured by:
Unique Pharmaceutical Laboratories
(A Div. of J. B. Chemicals & Pharmaceuticals Ltd.)
Mumbai 400 030, India
ORG 12/23
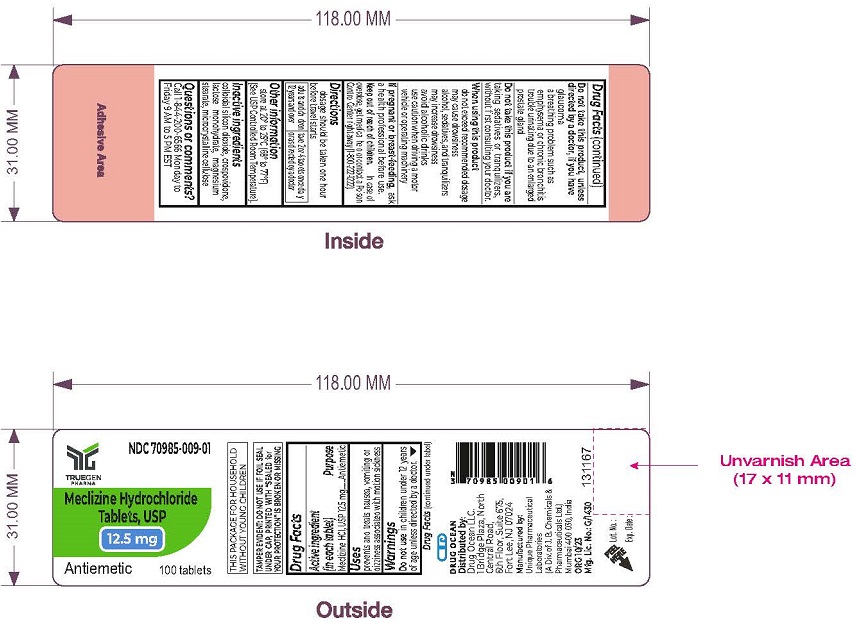
PRINCIPAL DISPLAY PANEL - 12.5 mg Tablet Label
NDC: 70985-009-01
Meclizine HCl
12.5 mg
100 Tablets
Distributed by:
Drug Ocean LLC,
1 Bridge Plaza, North Central Road,
6th Floor, Suite 675
Fort Lee, NJ 07024
Manufactured by:
Unique Pharmaceutical Laboratories
(A Div. of J. B. Chemicals & Pharmaceuticals Ltd.)
Mumbai 400 030, India
ORG 12/23
| MECLIZINE HCL
meclizine hydrochloride tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Drug Ocean LLC (080381835) |
| Registrant - Drug Ocean LLC (080381835) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Unique Pharmaceutical Laboratories | 650434645 | manufacture(70985-009) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.