Icosapent Ethyl by Hikma Pharmaceuticals USA Inc. / West-Ward Columbus Inc. ICOSAPENT ETHYL capsule
Icosapent Ethyl by
Drug Labeling and Warnings
Icosapent Ethyl by is a Prescription medication manufactured, distributed, or labeled by Hikma Pharmaceuticals USA Inc., West-Ward Columbus Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ICOSAPENT ETHYL CAPSULES safely and effectively. See full prescribing information for ICOSAPENT ETHYL CAPSULES.
ICOSAPENT ETHYL capsules, for oral use
Initial U.S. Approval: 2012INDICATIONS AND USAGE
Icosapent ethyl capsules are an ethyl ester of eicosapentaenoic acid (EPA) indicated:
- as an adjunct to diet to reduce triglyceride (TG) levels in adult patients with severe (≥ 500 mg/dL) hypertriglyceridemia. (1)
Limitations of Use:
- The effect of icosapent ethyl on the risk for pancreatitis in patients with severe hypertriglyceridemia has not been determined. (1)
DOSAGE AND ADMINISTRATION
- Assess lipid levels before initiating therapy. Identify other causes of high triglyceride levels and manage as appropriate. (2.1)
- Patients should engage in appropriate nutritional intake and physical activity before receiving icosapent ethyl capsules, which should continue during treatment. (2.1)
-
The daily dose of icosapent ethyl is 4 grams per day taken as either
- o four 0.5 gram capsules twice daily with food or
- o two 1-gram capsules twice daily with food. (2.2)
- Advise patients to swallow capsules whole. Do not break open, crush, dissolve, or chew icosapent ethyl capsules. (2.2)
DOSAGE FORMS AND STRENGTHS
Capsules: 0.5 gram and 1 gram (3)
CONTRAINDICATIONS
Icosapent ethyl is contraindicated in patients with known hypersensitivity (e.g., anaphylactic reaction) to icosapent ethyl or any of its components. (4)
WARNINGS AND PRECAUTIONS
Atrial Fibrillation/Flutter: Icosapent ethyl was associated with an increased risk of atrial fibrillation or atrial flutter requiring hospitalization in a double-blind, placebo-controlled trial. The incidence of atrial fibrillation was greater in patients with a previous history of atrial fibrillation or atrial flutter. (5.1)
Potential for Allergic Reactions in Patients with Fish Allergy: Icosapent ethylcontains ethyl esters of the omega-3 fatty acid, eicosapentaenoic acid (EPA), obtained from the oil of fish. It is not known whether patients with allergies to fish and/or shellfish are at increased risk of an allergic reaction to icosapent ethyl. Inform patients with known hypersensitivity to fish and/or shellfish about the potential for allergic reactions and advise them to discontinue icosapent ethyl and seek medical attention if any reactions occur. (5.2)
Bleeding: Icosapent ethylwas associated with an increased risk of bleeding in a double-blind, placebo-controlled trial. The incidence of bleeding was greater in patients receiving concomitant antithrombotic medications, such as aspirin, clopidogrel, or warfarin. (5.3)
ADVERSE REACTIONS
Common adverse reactions (incidence ≥3% and ≥1% more frequent than placebo): musculoskeletal pain, peripheral edema, constipation, gout, and atrial fibrillation (6.1)
Common adverse reactions in the hypertriglyceridemia trials (incidence ≥1% more frequent than placebo): arthralgia and oropharyngeal pain. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Hikma Pharmaceuticals USA Inc. at 1-800-962-8364 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Increased Bleeding Risk with Anticoagulants and Antiplatelet Agents: Some published studies with omega-3 fatty acids have demonstrated prolongation of bleeding time. Monitor patients receiving icosapent ethyl capsules and concomitant anticoagulants and/or antiplatelet agents for bleeding. (7)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 3/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Prior to Initiation of Icosapent Ethyl
2.2 Dosage and Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Atrial Fibrillation/Flutter
5.2 Potential for Allergic Reactions in Patients with Fish Allergy
5.3 Bleeding
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Increased Bleeding Risk with Anticoagulants and Antiplatelet Agents
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.7 Hepatic Impairment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.2 Severe Hypertriglyceridemia
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Prior to Initiation of Icosapent Ethyl
- Assess lipid levels before initiating therapy. Identify other causes (e.g., diabetes mellitus, hypothyroidism, or medications) of high triglyceride levels and manage as appropriate.
- Patients should engage in appropriate nutritional intake and physical activity before receiving icosapent ethyl, which should continue during treatment with icosapent ethyl.
2.2 Dosage and Administration
-
The daily dose of icosapent ethyl is 4 grams per day taken as either:
- o four 0.5 gram capsules twice daily with food; or as
- o two 1 gram capsules twice daily with food.
- Advise patients to swallow icosapent ethyl capsules whole. Do not break open, crush, dissolve, or chew icosapent ethyl capsules.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Atrial Fibrillation/Flutter
Icosapent ethyl is associated with an increased risk of atrial fibrillation or atrial flutter requiring hospitalization. In a double-blind, placebo-controlled trial of 8,179 subjects, adjudicated atrial fibrillation or atrial flutter requiring hospitalization for 24 or more hours occurred in 127 (3%) patients treated with icosapent ethyl compared to 84 (2%) patients receiving placebo [HR= 1.5 (95% CI 1.14, 1.98)]. The incidence of atrial fibrillation was greater in patients with a previous history of atrial fibrillation or atrial flutter.
5.2 Potential for Allergic Reactions in Patients with Fish Allergy
Icosapent ethyl contains ethyl esters of the omega-3 fatty acid, eicosapentaenoic acid (EPA), obtained from the oil of fish. It is not known whether patients with allergies to fish and/or shellfish are at increased risk of an allergic reaction to icosapent ethyl. Inform patients with known hypersensitivity to fish and/or shellfish about the potential for allergic reactions to icosapent ethyl and advise them to discontinue icosapent ethyl and seek medical attention if any reactions occur.
5.3 Bleeding
Icosapent ethyl is associated with an increased risk of bleeding. In a double-blind, placebo-controlled trial of 8,179 patients, 482 (12%) patients receiving icosapent ethyl experienced a bleeding event compared to 404 (10%) patients receiving placebo. Serious bleeding events occurred in 111 (3%) of patients on icosapent ethyl vs. 85 (2%) of patients receiving placebo. The incidence of bleeding was greater in patients receiving concomitant antithrombotic medications, such as aspirin, clopidogrel, or warfarin.
-
6 ADVERSE REACTIONS
The following important adverse reactions are described below and elsewhere in the labeling:
- Atrial Fibrillation or Atrial Flutter [see Warnings and Precautions (5.1)]
- Potential for Allergic Reactions in Patients with Fish Allergy [see Warnings and Precautions (5.2)]
- Bleeding [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Common adverse reactions (incidence ≥3% on icosapent ethyl and ≥1% more frequent than placebo) included musculoskeletal pain, peripheral edema, constipation, gout, and atrial fibrillation.
Hypertriglyceridemia Trials
In two randomized, double-blind, placebo-controlled trials in patients with triglyceride levels between 200 and 2000 mg/dL treated for 12 weeks, adverse reactions reported with icosapent ethyl at an incidence ≥1% more frequent than placebo based on pooled data included arthralgia and oropharyngeal pain.
6.2 Postmarketing Experience
Additional adverse reactions have been identified during post-approval use of icosapent ethyl. Because these reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Diarrhea
- Blood triglycerides increased
- Abdominal discomfort
- Pain in the extremities
-
7 DRUG INTERACTIONS
7.1 Increased Bleeding Risk with Anticoagulants and Antiplatelet Agents
Some published studies with omega-3 fatty acids have demonstrated prolongation of bleeding time. The prolongation of bleeding time reported in those studies has not exceeded normal limits and did not produce clinically significant bleeding episodes. Monitor patients receiving icosapent ethyl and concomitant anticoagulants and/or antiplatelet agents for bleeding.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
The available data from published case reports and the pharmacovigilance database on the use of icosapent ethyl in pregnant women are insufficient to identify a drug-associated risk for major birth defects, miscarriage or adverse maternal or fetal outcomes. In animal reproduction studies in pregnant rats, non-dose-related imbalances for some minor developmental findings were observed with oral administration of icosapent ethyl during organogenesis at exposures that were equivalent to the clinical exposure at the human dose of 4 g/day, based on body surface area comparisons. In a study in pregnant rabbits orally administered icosapent ethyl during organogenesis, there were no clinically relevant adverse developmental effects at exposures that were 5 times the clinical exposure, based on body surface area comparisons (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
In pregnant rats given oral gavage doses of 0.3, 1 and 2 g/kg/day icosapent ethyl from gestation through organogenesis all drug treated groups had non-dose-related imbalances in visceral and skeletal findings, including 13th reduced ribs, additional liver lobes, testes medially displaced and/or not descended, at human systemic exposures following a maximum oral dose of 4 g/day based on body surface comparisons.
In a multigenerational developmental study in pregnant rats given doses of 0.3, 1, 3 g/kg/day icosapent ethyl by oral gavage from gestation day 7-17, icosapent ethyl did not affect viability in fetuses (F1 or F2). Non-dose-related imbalances in findings of absent optic nerves and unilateral testes atrophy at human exposures based on the maximum dose of 4 g/day and on body surface area comparisons. Additional variations consisting of early incisor eruption and increased percent cervical ribs were observed at the same exposures. Pups from high dose treated dams exhibited decreased copulation rates, delayed estrus, decreased implantations and decreased surviving fetuses (F2) suggesting potential multigenerational effects of icosapent ethyl at 7 times human systemic exposure following 4 g/day dose based on body surface area comparisons across species.
In pregnant rabbits given oral gavage doses of 0.1, 0.3, and 1 g/kg/day icosapent ethyl from gestation through organogenesis, a decrease in body weight and food consumption was observed at the high dose of 1 g/kg/day (5 times the human exposure at the maximum dose of 4 g/day, based on body surface area comparisons). Slight increases in resorbed and dead fetuses were noted in the 1 g/kg/day group, but these were not significantly different from the control group. There were no differences between the icosapent ethyl groups and control group as to the number of corpora lutea, number of implantations, number of surviving fetuses, sex ratio, body weight of female fetuses or placental weight. There were no treatment-related malformations or skeletal anomalies.
In pregnant rats given icosapent ethyl from gestation day 17 through lactation day 20 at 0.3, 1, 3 g/kg/day no adverse maternal or developmental effects were observed. However, complete litter loss (not dose-related) was noted in 2/23 litters at the low dose and 1/23 mid-dose dams by post-natal day 4 at human exposures at a maximum dose of 4 g/day, based on body surface area comparisons.
8.2 Lactation
Risk Summary
Published studies have detected omega-3 fatty acids, including EPA, in human milk. Lactating women receiving oral omega-3 fatty acids for supplementation have resulted in higher levels of omega-3 fatty acids in human milk. There are no data on the effects of omega-3 fatty acid ethyl esters on the breastfed infant or on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for icosapent ethyl and any potential adverse effects on the breastfed child from icosapent ethyl or from the underlying maternal condition.
8.5 Geriatric Use
Of the total number of patients in well-controlled clinical studies of icosapent ethyl, 45% were 65 years of age and over. No overall differences in safety or effectiveness were observed between these patients and younger groups. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
-
11 DESCRIPTION
Icosapent ethyl, a lipid-regulating agent, is supplied as a 0.5 gram and 1 gram, liquid-filled soft gelatin capsule for oral administration.
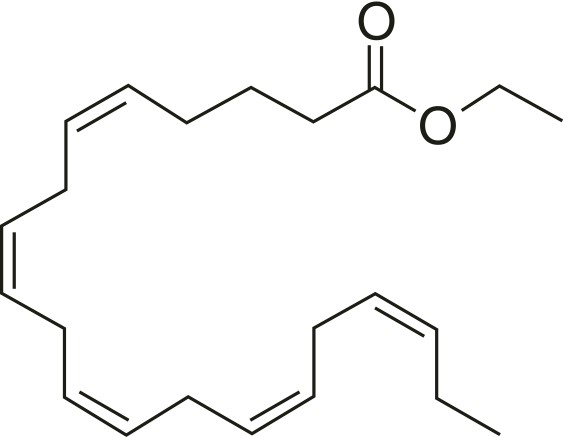
Each icosapent ethyl capsule contains either 0.5 grams of icosapent ethyl or 1 gram of icosapent ethyl. Icosapent ethyl is an ethyl ester of the omega-3 fatty acid eicosapentaenoic acid (EPA). The empirical formula of icosapent ethyl is C22H34O2 and the molecular weight is 330.5. The chemical name for icosapent ethyl is ethyl all-cis-5,8,11,14,17-icosapentaenoate with the following chemical structure:
Each capsule contains the following inactive ingredients: gelatin, glycerin, purified water, sorbitol, sorbitan and tocopherol. The monogramming ink ingredients contain: ammonium hydroxide, iron oxide black, isopropyl alcohol, macrogol, polyvinyl acetate phthalate, propylene glycol, purified water and SDA alcohol (ethanol and ethyl acetate).
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Studies suggest that EPA reduces hepatic very low-density lipoprotein triglycerides (VLDL-TG) synthesis and/or secretion and enhances TG clearance from circulating VLDL particles. Potential mechanisms of action include increased β-oxidation; inhibition of acyl-CoA:1,2-diacylglycerol acyltransferase (DGAT); decreased lipogenesis in the liver; and increased plasma lipoprotein lipase activity.
12.2 Pharmacodynamics
In a 12-week, dose-ranging study in patients with severe hypertriglyceridemia, icosapent ethyl 4 grams per day reduced median TG from baseline relative to placebo [see Clinical Studies (14)].
12.3 Pharmacokinetics
Absorption
After oral administration, icosapent ethyl is de-esterified during the absorption process and the active metabolite EPA is absorbed in the small intestine and enters the systemic circulation mainly via the thoracic duct lymphatic system. Peak plasma concentrations of EPA were reached approximately 5 hours following oral doses of icosapent ethyl.
Icosapent ethyl was administered with or following a meal in all clinical studies; no food effect studies were performed. Take icosapent ethyl with or following a meal.
Distribution
The mean volume of distribution at steady state of EPA is approximately 88 liters. The majority of EPA circulating in plasma is incorporated in phospholipids, triglycerides and cholesteryl esters, and <1% is present as the unesterified fatty acid. Greater than 99% of unesterified EPA is bound to plasma proteins.
Elimination
Metabolism
EPA is mainly metabolized by the liver via beta-oxidation similar to dietary fatty acids. Beta oxidation splits the long carbon chain of EPA into acetyl Coenzyme A, which is converted into energy via the Krebs cycle. Cytochrome P450-mediated metabolism is a minor pathway of elimination of EPA.
Excretion
The total plasma clearance of EPA at steady state is 684 mL/hr. The plasma elimination half-life (t1/2) of EPA is approximately 89 hours. Icosapent ethyl does not undergo renal excretion.
Specific Populations
Gender
When administered icosapent ethyl in clinical trials, plasma total EPA concentrations did not differ significantly between men and women.
Pediatric
The pharmacokinetics of icosapent ethyl has not been studied in pediatric patients.
Hepatic or Renal Impairment
Icosapent ethyl has not been studied in patients with renal or hepatic impairment.
Drug Interaction Studies
Omeprazole: In a drug-drug interaction study with 28 healthy adult subjects, icosapent ethyl 4 g/day at steady-state did not significantly change the steady-state AUCτ or Cmax of omeprazole when co-administered at 40 mg/day to steady-state.
Rosiglitazone: In a drug-drug interaction study with 28 healthy adult subjects, icosapent ethyl 4 g/day at steady-state did not significantly change the single dose AUC or Cmax of rosiglitazone at 8 mg.
Warfarin: In a drug-drug interaction study with 25 healthy adult subjects, icosapent ethyl 4 g/day at steady-state did not significantly change the single dose AUC or Cmax of R- and S-warfarin or the anti-coagulation pharmacodynamics of warfarin when co-administered as racemic warfarin at 25 mg.
Atorvastatin: In a drug-drug interaction study of 26 healthy adult subjects, icosapent ethyl 4 g/day at steady-state did not significantly change the steady-state AUCτ or Cmax of atorvastatin, 2-hydroxyatorvastatin, or 4-hydroxyatorvastatin when co-administered with atorvastatin 80 mg/day at steady-state.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 2-year rat carcinogenicity study with oral gavage doses of 0.09, 0.27, and 0.91 g/kg/day icosapent ethyl, respectively, males did not exhibit drug-related neoplasms. Hemangiomas and hemangiosarcomas of the mesenteric lymph node, the site of drug absorption, were observed in females at clinically relevant exposures based on body surface area comparisons across species relative to the maximum clinical dose of 4 g/day. Overall incidence of hemangiomas and hemangiosarcomas in all vascular tissues did not increase with treatment.
In a 6-month carcinogenicity study in Tg.rasH2 transgenic mice with oral gavage doses of 0.5, 1, 2, and 4.6 g/kg/day icosapent ethyl, drug-related incidences of benign squamous cell papilloma in the skin and subcutis of the tail was observed in high dose male mice. The papillomas were considered to develop secondary to chronic irritation of the proximal tail associated with fecal excretion of oil and therefore not clinically relevant. Drug-related neoplasms were not observed in female mice.
Icosapent ethyl was not mutagenic with or without metabolic activation in the bacterial mutagenesis (Ames) assay or in the in vivo mouse micronucleus assay. A chromosomal aberration assay in Chinese Hamster Ovary (CHO) cells was positive for clastogenicity with and without metabolic activation.
In an oral gavage rat fertility study, ethyl-EPA, administered at doses of 0.3, 1, and 3 g/kg/day to male rats for 9 weeks before mating and to female rats for 14 days before mating through day 7 of gestation, increased anogenital distance in female pups and increased cervical ribs were observed at 3 g/kg/day (7 times human systemic exposure with 4 g/day clinical dose based on a body surface area comparison).
-
14 CLINICAL STUDIES
14.2 Severe Hypertriglyceridemia
The effects of icosapent ethyl 4 grams per day were assessed in a randomized, placebo-controlled, double-blind, parallel-group study of adult patients (76 on icosapent ethyl, 75 on placebo) with severe hypertriglyceridemia. Patients whose baseline TG levels were between 500 and 2,000 mg/dL were enrolled in this study for 12 weeks. The median baseline TG and LDL-C levels in these patients were 684 mg/dL and 86 mg/dL, respectively. Median baseline HDL-C level was 27 mg/dL. The randomized population in this study was mostly Caucasian (88%) and male (76%). The mean age was 53 years and the mean body mass index was 31 kg/m2. Twenty-five percent of patients were on concomitant statin therapy, 28% were diabetics, and 39% of the patients had TG levels >750 mg/dL.
The changes in the major lipoprotein lipid parameters for the groups receiving icosapent ethyl or placebo are shown in Table 2.
Table 2. Median Baseline and Percent Change from Baseline in Lipid Parameters in Patients with Severe Hypertriglyceridemia (≥500 mg/dL) - * p-value < 0.001 (primary efficacy endpoint)
- † p-value < 0.05 (key secondary efficacy endpoints determined to be statistically significant according to the pre-specified multiple comparison procedure)
Parameter
Icosapent Ethyl 4 g/day
N=76
Placebo
N=75
Difference
(95% Confidence Interval)
Baseline
% Change
Baseline
% Change
TG (mg/dL)
680
-27
703
+10
-33*, (-47, -22)
LDL-C (mg/dL)
91
-5
86
-3
-2 (-13, +8)
Non-HDL-C (mg/dL)
225
-8
229
+8
-18 (-25, -11)
TC (mg/dL)
254
-7
256
+8
-16 (-22, -11)
HDL-C (mg/dL)
27
-4
27
0
-4 (-9, +2)
VLDL-C (mg/dL)
123
-20
124
+14
-29† (-43, -14)
Apo B (mg/dL)
121
-4
118
+4
-9† (-14, -3)
% Change = Median Percent Change from Baseline
Difference = Median of [Icosapent ethyl % Change – Placebo % Change] (Hodges-Lehmann Estimate)
p-values from Wilcoxon rank-sum test
Icosapent ethyl 4 grams per day reduced median TG, VLDL-C, and Apo B levels from baseline relative to placebo. The reduction in TG observed with icosapent ethyl was not associated with elevations in LDL-C levels relative to placebo.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Icosapent Ethyl Capsules
1 gram capsules are supplied as a clear, oblong capsule filled with colorless to pale yellow oily liquid and printed with “54 648” in black ink on one side.
NDC: 0054-0508-23: Bottle of 120 Capsules
0.5 gram capsules are supplied as a clear, oval capsule filled with colorless to pale yellow oily liquid, and printed with ‘109” in black ink on one side.
NDC: 0054-0621-27: Bottle of 240 Capsules
Store at 20° to 25° C (68° to 77°F). [See USP Controlled Room Temperature.] Keep out of reach of children.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling before starting icosapent ethyl (Patient Information).
Inform patients that icosapent ethyl may increase their risk for atrial fibrillation or atrial flutter [see Warnings and Precautions (5.1)].
Inform patients with known hypersensitivity to fish and/or shellfish about the potential for allergic reactions to icosapent ethyl and advise them to discontinue icosapent ethyl and seek medical attention if any reactions occur [see Warnings and Precautions (5.2)].
Inform patients that icosapent ethyl may increase their risk for bleeding, especially if they are receiving other antithrombotic agents [see Warnings and Precautions (5.3)].
Advise patients to swallow icosapent ethyl capsules whole. Do not break open, crush, dissolve, or chew icosapent ethyl [see Dosage and Administration (2.2)].
Instruct patients to take icosapent ethyl as prescribed. If a dose is missed, patients should take it as soon as they remember. However, if they miss one day of icosapent ethyl, they should not double the dose when they take it.
For more information about icosapent ethyl, please call Hikma Pharmaceuticals USA Inc. at 1-800-962-8364.
Manufactured by:
Catalent Pharma Solutions, LLC.
St. Petersburg, Florida 33716
Distributed by:
Hikma Pharmaceuticals USA Inc.
Berkeley Heights, NJ 07922
C50001301/01
Revised October 2022
-
PATIENT INFORMATION
Icosapent Ethyl
Capsules
(eye koe’ sa pent eth’ il)
Rx only
What is icosapent ethyl?
Icosapent ethyl is a prescription medicine used:
- along with a low-fat and low-cholesterol diet to lower high levels of triglycerides (fats) in adults.
It is not known if icosapent ethyl changes your risk of having inflammation of your pancreas (pancreatitis).
It is not known if icosapent ethyl is safe and effective in children.
Do not take icosapent ethyl capsules if you are allergic to icosapent ethyl or any of the ingredients in icosapent ethyl capsules. See the end of this leaflet for a complete list of ingredients in icosapent ethyl capsules.
Before taking icosapent ethyl, tell your doctor about all of your medical conditions, including if you:
- have diabetes.
- have a low thyroid problem (hypothyroidism).
- have a liver problem.
- have a pancreas problem.
- are allergic to fish or shellfish. It is not known if people who are allergic to fish or shellfish are also allergic to icosapent ethyl.
- are pregnant, or planning to become pregnant. It is not known if icosapent ethyl will harm your unborn baby.
- are breastfeeding or plan to breastfeed. Icosapent ethyl can pass into your breast milk, and may harm your baby. Talk to your doctor about the best way to feed your baby if you take icosapent ethyl.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and dietary or herbal supplements.
Icosapent ethyl can interact with certain other medicines that you are taking.
Especially tell your doctor if you take medicines that affect your blood clotting (anticoagulants or blood thinners).
How should I take icosapent ethyl?
- Take icosapent ethyl exactly as your doctor tells you to take it.
- Do not change your dose or stop taking icosapent ethyl without talking to your doctor.
-
Do not take more capsules than what is prescribed by your doctor.
- o If you are prescribed the 0.5-gram capsules, you should not take more than 8 capsules each day with food.
- o If you are prescribed the 1-gram capsules, you should not take more than 4 capsules each day with food.
- Take icosapent ethyl capsules whole. Do not break, crush, dissolve, or chew icosapent ethyl capsules before swallowing.
- If you miss a dose of icosapent ethyl, take it as soon as you remember. However, if you miss one day of icosapent ethyl, do not double your dose when you take it.
- Your doctor may start you on a diet that is low in saturated fat, cholesterol, carbohydrates, and low in added sugars before giving you icosapent ethyl. Stay on this diet while taking icosapent ethyl.
- Your doctor may do blood tests to check your triglyceride and other lipid levels while you take icosapent ethyl.
What are the possible side effects of icosapent ethyl?
Icosapent ethyl may cause serious side effects, including:
- Heart rhythm problems (atrial fibrillation and atrial flutter). Heart rhythm problems which can be serious and cause hospitalization have happened in people who take icosapent ethyl, especially in people who have heart (cardiovascular) disease or diabetes with a risk factor for heart (cardiovascular) disease, or who have had heart rhythm problems in the past. Tell your doctor if you get any symptoms of heart rhythm problems such as feeling as if your heart is beating fast and irregular, lightheadedness, dizziness, shortness of breath, chest discomfort, or you faint.
- Possible allergic reactions if you are allergic to fish or shellfish. Stop taking icosapent ethyl and tell your doctor right away or get emergency medical help if you have any signs or symptoms of an allergic reaction.
- Bleeding. Serious bleeding can happen in people who take icosapent ethyl. Your risk of bleeding may increase if you are also taking a blood thinner medicine.
If you have liver problems and are taking icosapent ethyl, your doctor should do blood tests during treatment.
The most common side effects of icosapent ethyl include:
- Muscle and joint pain.
- Swelling of the hands, legs, or feet.
- Constipation
- Gout
- Heart rhythm problems (atrial fibrillation).
These are not all the possible side effects of icosapent ethyl. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store Icosapent Ethyl Capsules?
- Store icosapent ethyl at room temperature between 68° to 77° F (20° to 25° C).
- Safely throw away medicine that is out of date or no longer needed.
Keep icosapent ethyl and all medicine out of the reach of children.
General information about the safe and effective use of icosapent ethyl.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use icosapent ethyl for a condition for which it was not prescribed. Do not give icosapent ethyl to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about icosapent ethyl that is written for health professionals.
What are the ingredients in Icosapent Ethyl Capsules?
Active ingredient: icosapent ethyl
Inactive ingredients: gelatin, glycerin, purified water, sorbitol, sorbitan and tocopherol. The monogramming ink ingredients contain: ammonium hydroxide, iron oxide black, isopropyl alcohol, macrogol, polyvinyl acetate phthalate, propylene glycol, purified water and SDA alcohol (ethanol and ethyl acetate).
This Patient Information has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Catalent Pharma Solutions, LLC.
St. Petersburg, Florida 33716
Distributed by:
Hikma Pharmaceuticals USA Inc.
Berkeley Heights, NJ 07922
C50001301/01
Revised October 2022
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
- Package/Label Display Panel
-
INGREDIENTS AND APPEARANCE
ICOSAPENT ETHYL
icosapent ethyl capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0054-0508 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ICOSAPENT ETHYL (UNII: 6GC8A4PAYH) (ICOSAPENT - UNII:AAN7QOV9EA) ICOSAPENT ETHYL 1 g Inactive Ingredients Ingredient Name Strength GELATIN, UNSPECIFIED (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) SORBITOL (UNII: 506T60A25R) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) 2 mg AMMONIA (UNII: 5138Q19F1X) FERROSOFERRIC OXIDE (UNII: XM0M87F357) ISOPROPYL ALCOHOL (UNII: ND2M416302) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POLYVINYL ACETATE PHTHALATE (UNII: 58QVG85GW3) ALCOHOL (UNII: 3K9958V90M) ETHYL ACETATE (UNII: 76845O8NMZ) SORBITAN (UNII: 6O92ICV9RU) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) Product Characteristics Color YELLOW Score no score Shape CAPSULE Size 25mm Flavor Imprint Code 54;648 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0054-0508-23 120 in 1 BOTTLE; Type 0: Not a Combination Product 11/04/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA209457 11/04/2020 ICOSAPENT ETHYL
icosapent ethyl capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0054-0621 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ICOSAPENT ETHYL (UNII: 6GC8A4PAYH) (ICOSAPENT - UNII:AAN7QOV9EA) ICOSAPENT ETHYL 0.5 g Inactive Ingredients Ingredient Name Strength GELATIN, UNSPECIFIED (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) SORBITOL (UNII: 506T60A25R) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) 1 mg AMMONIA (UNII: 5138Q19F1X) FERROSOFERRIC OXIDE (UNII: XM0M87F357) ISOPROPYL ALCOHOL (UNII: ND2M416302) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POLYVINYL ACETATE PHTHALATE (UNII: 58QVG85GW3) ALCOHOL (UNII: 3K9958V90M) ETHYL ACETATE (UNII: 76845O8NMZ) SORBITAN (UNII: 6O92ICV9RU) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) Product Characteristics Color YELLOW Score no score Shape OVAL Size 15mm Flavor Imprint Code 109 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0054-0621-27 240 in 1 BOTTLE; Type 0: Not a Combination Product 03/09/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA209457 11/04/2020 Labeler - Hikma Pharmaceuticals USA Inc. (080189610)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.