FLUDEOXYGLUCOSE F18 injection
Fludeoxyglucose by
Drug Labeling and Warnings
Fludeoxyglucose by is a Prescription medication manufactured, distributed, or labeled by Houston Cyclotron Partners LP dba Cyclotope. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use Fludeoxyglucose F18 Injection safely and effectively. See full prescribing information for Fludeoxyglucose F18 Injection.
Fludeoxyglucose F18 Injection
Initial U.S. Approval: 2005DOSAGE FORMS AND STRENGTHS
Multiple-dose glass vial containing 0.74-18.5 GBq/mL (20-500 mCi/mL) of Fludeoxyglucose F18 Injection and 4.5 mg of sodium chloride in citrate buffer (approximately 16 – 17 mL volume), for intravenous administration (3).
ADVERSE REACTIONS
Hypersensitivity reactions have occurred; have emergency resuscitation equipment and personnel immediately available (6).
To report SUSPECTED ADVERSE REACTIONS, contact CYCLOTOPE at 1-713-747-5686 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Revised: 8/2012
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
3 DOSAGE FORMS AND STRENGTHS
6 ADVERSE REACTIONS
11 DESCRIPTION
11.1 Chemical Characteristics
11.2 Physical Characteristics
16 HOW SUPPLIED / STORAGE AND DRUG HANDLING
- * Sections or subsections omitted from the full prescribing information are not listed.
- 3 DOSAGE FORMS AND STRENGTHS
- 6 ADVERSE REACTIONS
-
11 DESCRIPTION
11.1 Chemical Characteristics
Fludeoxyglucose F 18 Injection is a positron emitting radiopharmaceutical that is used for diagnostic purposes in conjunction with positron emission tomography (PET) imaging. The active ingredient 2-deoxy-2-[ 18F]fluoro-D-glucose has the molecular formula of C 6H 1118FO 5with a molecular weight of 181.26, and has the following chemical structure:
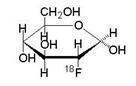
Fludeoxyglucose F 18 Injection is provided as a ready to use sterile, pyrogen free, clear, colorless citrate buffered solution. Each mL contains between 0.740 to 18.5 GBq (20.0-500 mCi) of 2-deoxy-2-[ 18F]fluoro-D-glucose at the EOS, 4.5 mg of sodium chloride in citrate buffer. The pH of the solution is between 4.5 and 7.5. The solution is packaged in a multiple-dose glass vial and does not contain any preservative.
11.2 Physical Characteristics
Fluorine F 18 has a physical half-life of 109.7 minutes and decays to Oxygen O 18 (stable) by positron decay. The principal photons useful for imaging are the dual 511 keV "annihilation" gamma photons, that are produced and emitted simultaneously in opposite direction when the positron interacts with an electron (Table 2).
Table 2: Principal Emission Data for Fluoride F18 Radiation/Emission % per Disintegration Mean Energy - * Produced by positron annihilation. From: Kocher, D.C. Radioactive Decay Tables DOE/TIC-I 1026, 89 (1981)
Positron (β+) 96.73 249.8 keV Gamma (±) * 193.46 511.0 keV The specific gamma ray constant (point source air kerma coefficient) for fluorine F 18 is 5.7 R/hr/mCi (1.35 x 10 -6 Gy/hr/kBq) at 1 cm. The half-value layer (HVL) for the 511 keV photons is 4 mm lead (Pb). The range of attenuation coefficients for this radionuclide as a function of lead shield thickness is shown in Table 3. For example, the interposition of an 8 mm thickness of Pb, with a coefficient of attenuation of 0.25, will decrease the external radiation by 75%.
Table 3:Radiation Attenuation of 511 keV Photons by Lead (Pb) Shielding Shield Thickness (Pb) mm Coefficient of Attenuation 0 0.00 4 0.50 8 0.25 13 0.10 26 0.01 39 0.001 52 0.0001 For use in correcting for physical decay of this radionuclide, the fractions remaining at selected intervals after calibration are shown in Table 4.
-
16 HOW SUPPLIED / STORAGE AND DRUG HANDLING
Fludeoxyglucose F 18 Injection is supplied in a multi-dose, capped 30 mL glass vial containing between 0.740 – 18.5 GBq/mL (20 - 500 mCi/mL), of no carrier added 2-deoxy-2-[F 18] fluoro-D-glucose, at end of synthesis, in approximately 16 - 17 mL. The contents of each vial are sterile, pyrogen-free and preservative-free.
NDC: 47584-001-01
Store the Fludeoxyglucose F 18 Injection vial upright in a lead shielded container at 20º to 25°C (68º to 77°F); excursions permitted to 15-30°C (59-86°F) [See USP Controlled Room Temperature].
Distribute, store and dispose of Fludeoxyglucose F 18 Injection in accordance with the regulations and a general license, or its equivalent, of an Agreement State or a Licensing State.
The expiration date and time are provided on the container label. Use Fludeoxyglucose F 18 Injection within 12 hours from the EOS time.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FLUDEOXYGLUCOSE F18
fludeoxyglucose f18 injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 47584-001 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FLUDEOXYGLUCOSE F-18 (UNII: 0Z5B2CJX4D) (FLUDEOXYGLUCOSE F-18 - UNII:0Z5B2CJX4D) FLUDEOXYGLUCOSE F-18 500 mCi in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 47584-001-01 16 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product 12/08/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA203665 12/08/2011 Labeler - Houston Cyclotron Partners LP dba Cyclotope (118258354) Establishment Name Address ID/FEI Business Operations Houston Cyclotron Partners LP dba Cyclotope 118258354 positron emission tomography drug production(47584-001)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.

