ESIKA PRO ADVANCE LASTING LONG-LASTING AND COMFORT SPF 20 (MARRON HAVANA) - BROWN- octinoxate lipstick ESIKA PRO ADVANCE LASTING LONG-LASTING AND COMFORT SPF 20 (NATURAL DUNE) - PINK- octinoxate lipstick ESIKA PRO ADVANCE LASTING LONG-LASTING AND COMFORT SPF 20 (MARRON FANATIC) - RED- octinoxate lipstick ESIKA PRO ADVANCE LASTING LONG-LASTING AND COMFORT SPF 20 (ROJO FIESTA) - RED- octinoxate lipstick ESIKA PRO ADVANCE LASTING LONG-LASTING AND COMFORT SPF 20 (MARRON BAMBU) - BROWN- octinoxate lipstick ESIKA PRO ADVANCE LASTING LONG-LASTING AND COMFORT SPF 20 (FUCSIA DELIRIO) - PINK- octinoxate lipstick ESIKA PRO ADVANCE LASTING LONG-LASTING AND COMFORT SPF 20 (ROJO PASION) - RED- octinoxate lipstick ESIKA PRO ADVANCE LASTING LONG-LASTING AND COMFORT SPF 20 (FUCSIA SUBLIME) - PINK- octinoxate lipstick ESIKA PRO ADVANCE LASTING LONG-LASTING AND COMFORT SPF 20 (ROSA FIORELLE) - PINK- octinoxate lipstick ESIKA PRO ADVANCE LASTING LONG-LASTING AND COMFORT SPF 20 (NARANJA OBSESION) - ORANGE- octinoxate lipstick ESIKA PRO ADVANCE LASTING LONG-LASTING AND COMFORT SPF 20 (BORGONA SWEET) - PINK- octinoxate lipstick ESIKA PRO ADVANCE LASTING LONG-LASTING AND COMFORT SPF 20 (VINO DESEO) - PURPLE- octinoxate lipstick ESIKA PRO ADVANCE LASTING LONG-LASTING AND COMFORT SPF 20 (FUCSIA TENTACION) - PINK- octinoxate lipstick ESIKA PRO ADVANCE LASTING LONG-LASTING AND COMFORT SPF 20- octinoxate kit
ESIKA PRO ADVANCE LASTING Long-lasting and comfort SPF 20 by
Drug Labeling and Warnings
ESIKA PRO ADVANCE LASTING Long-lasting and comfort SPF 20 by is a Otc medication manufactured, distributed, or labeled by Ventura Corporation LTD, Bel Star S.A. (Colombia). Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
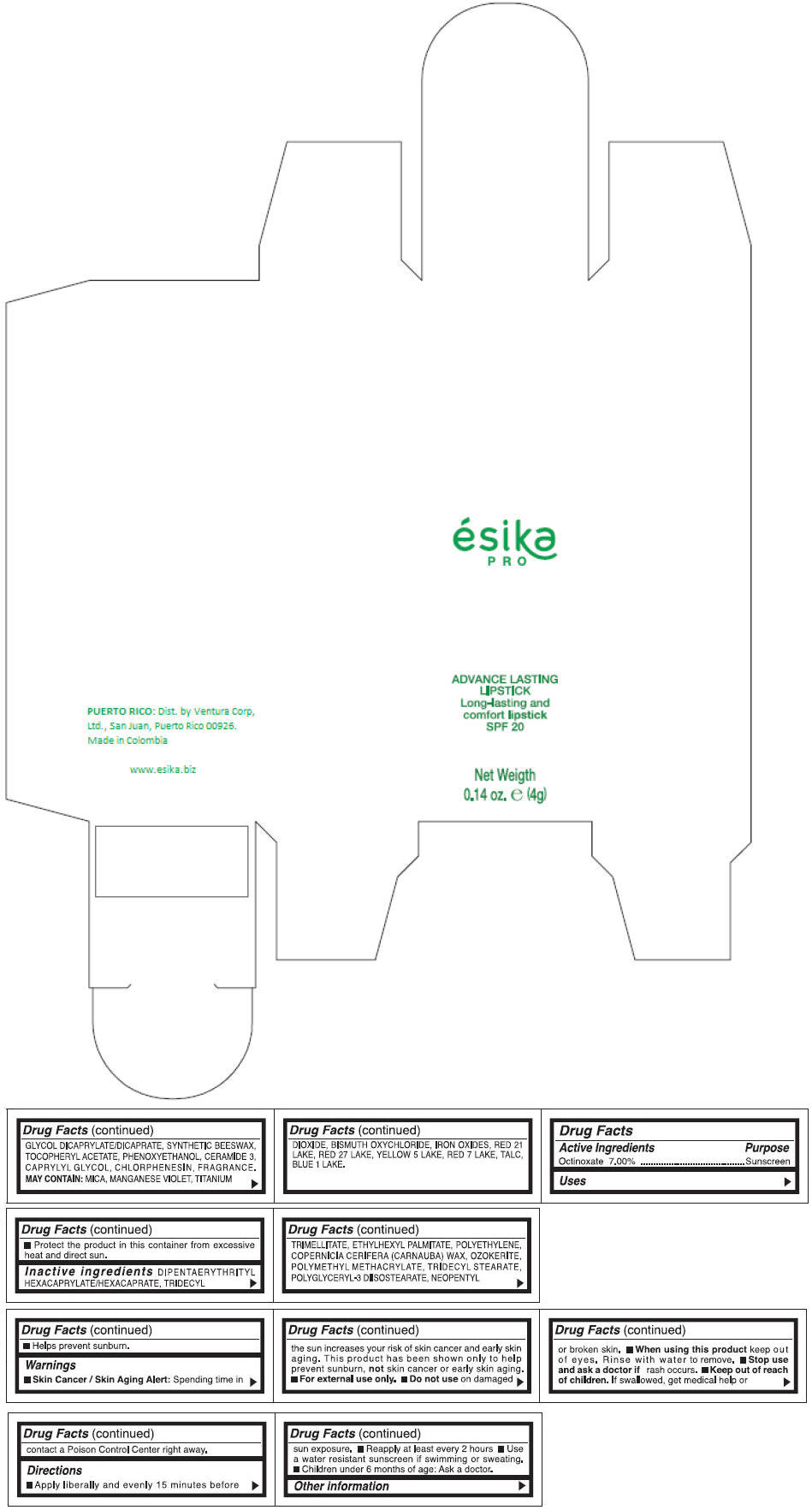
- Active Ingredients
- Purpose
- Uses
- Warnings
- Directions
- Other information
-
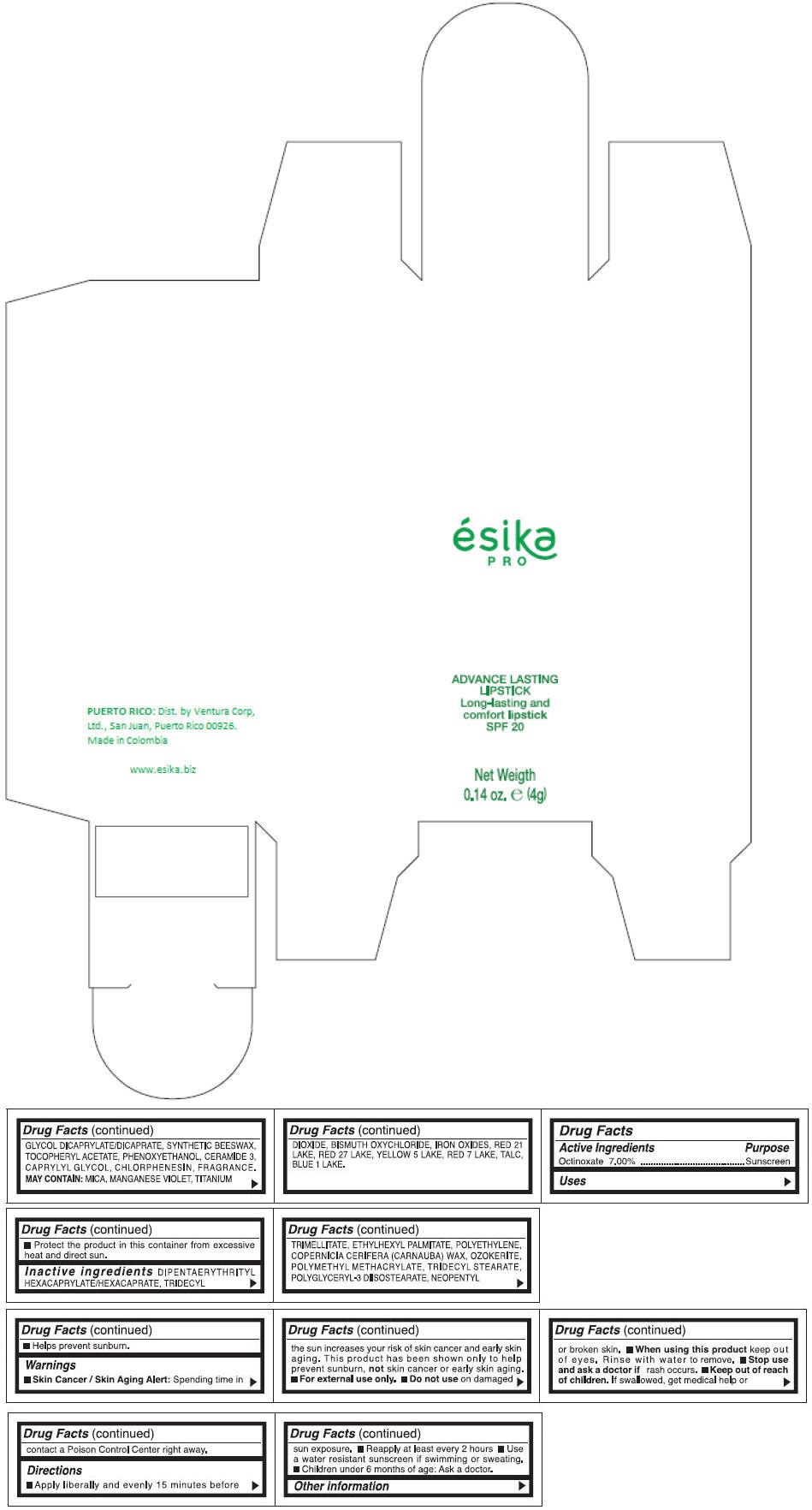
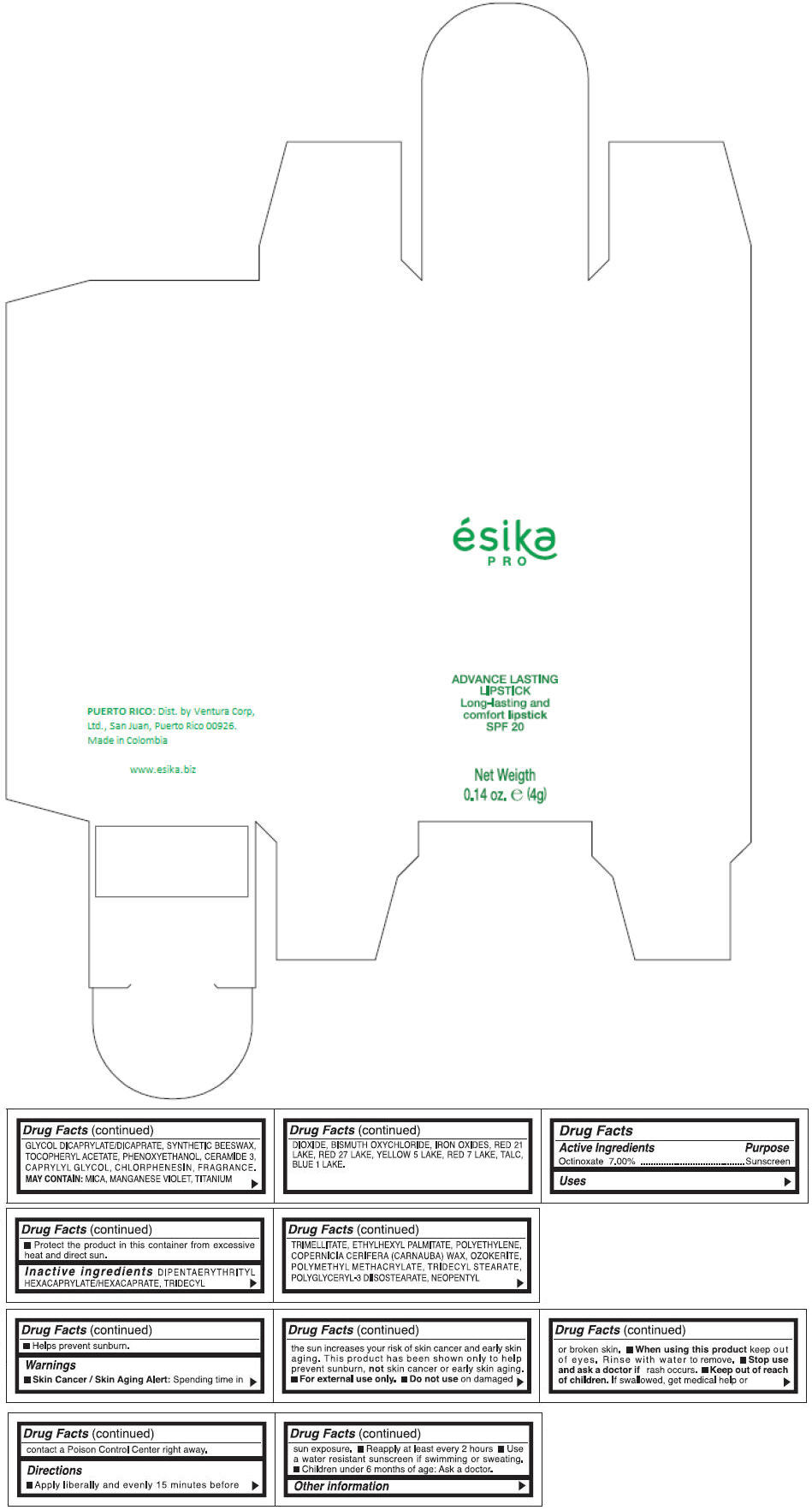
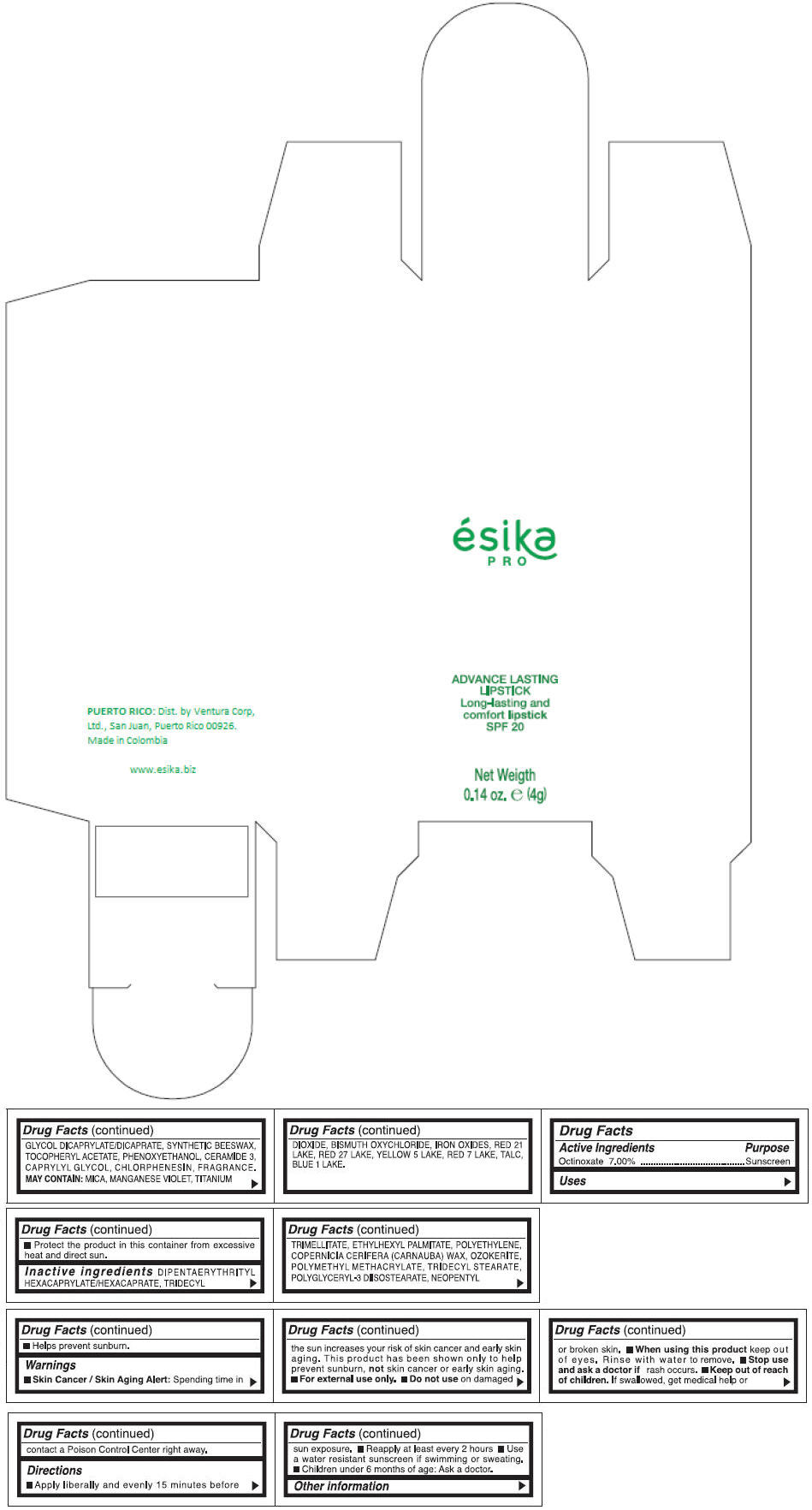
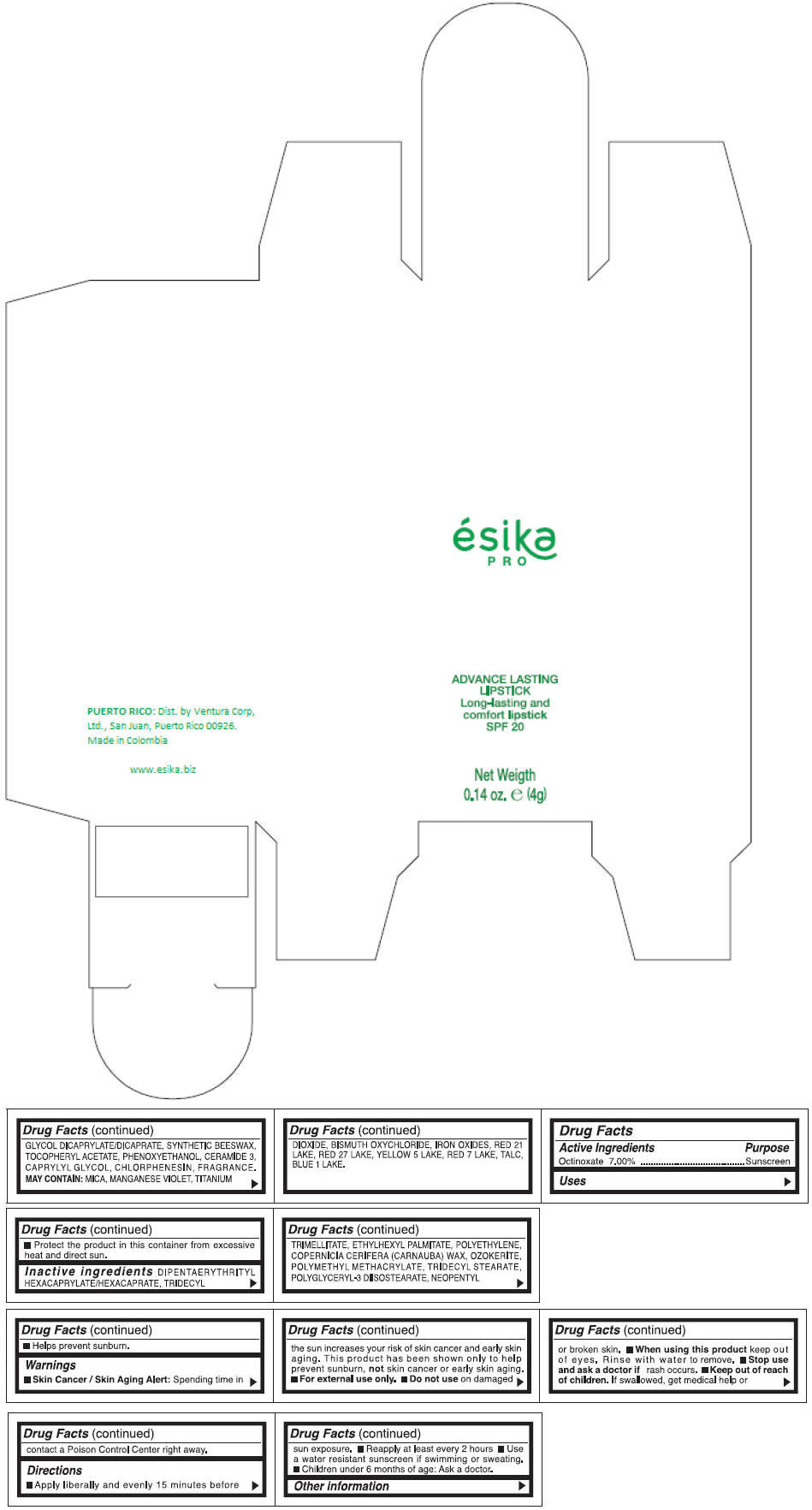
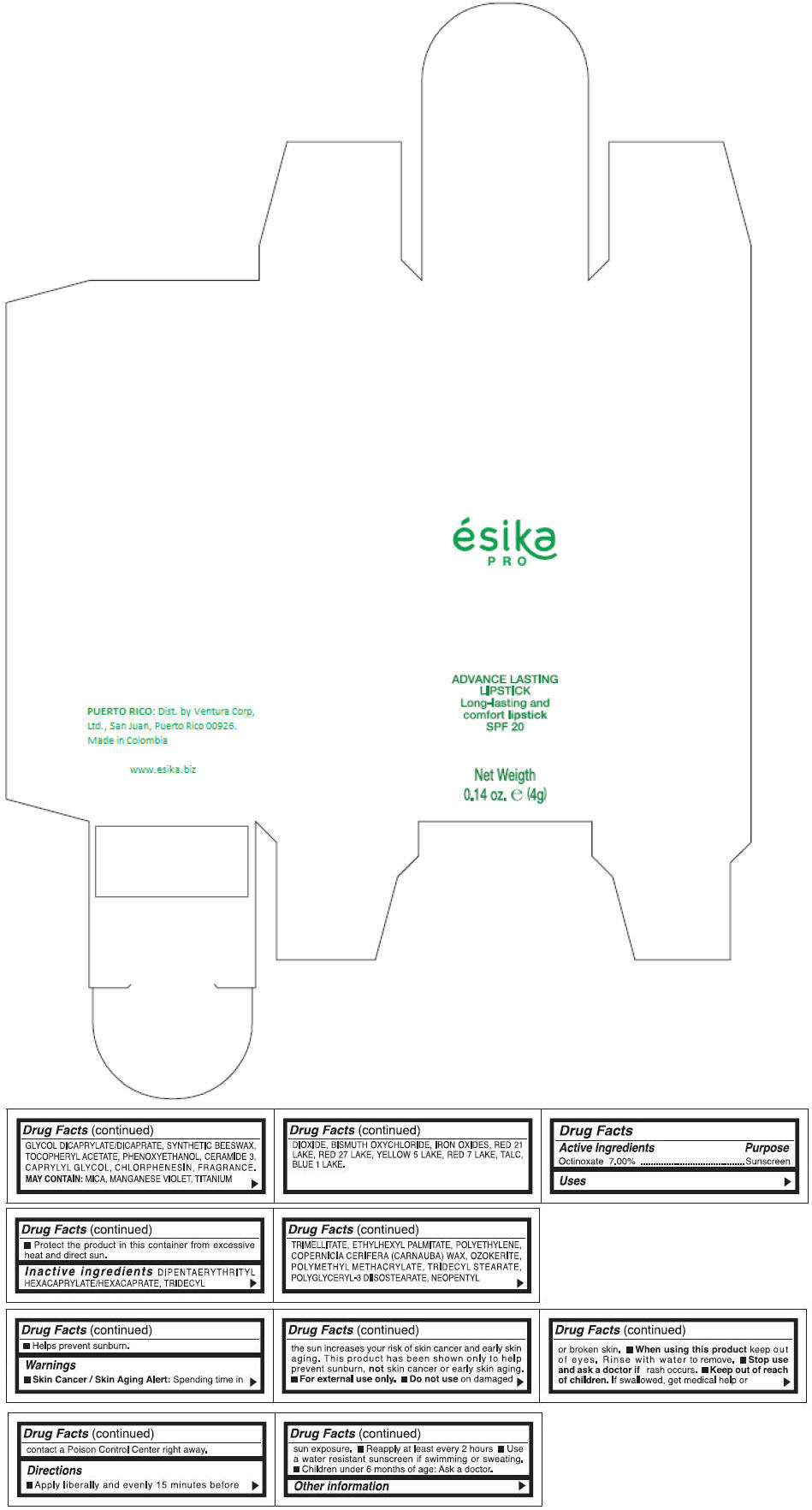
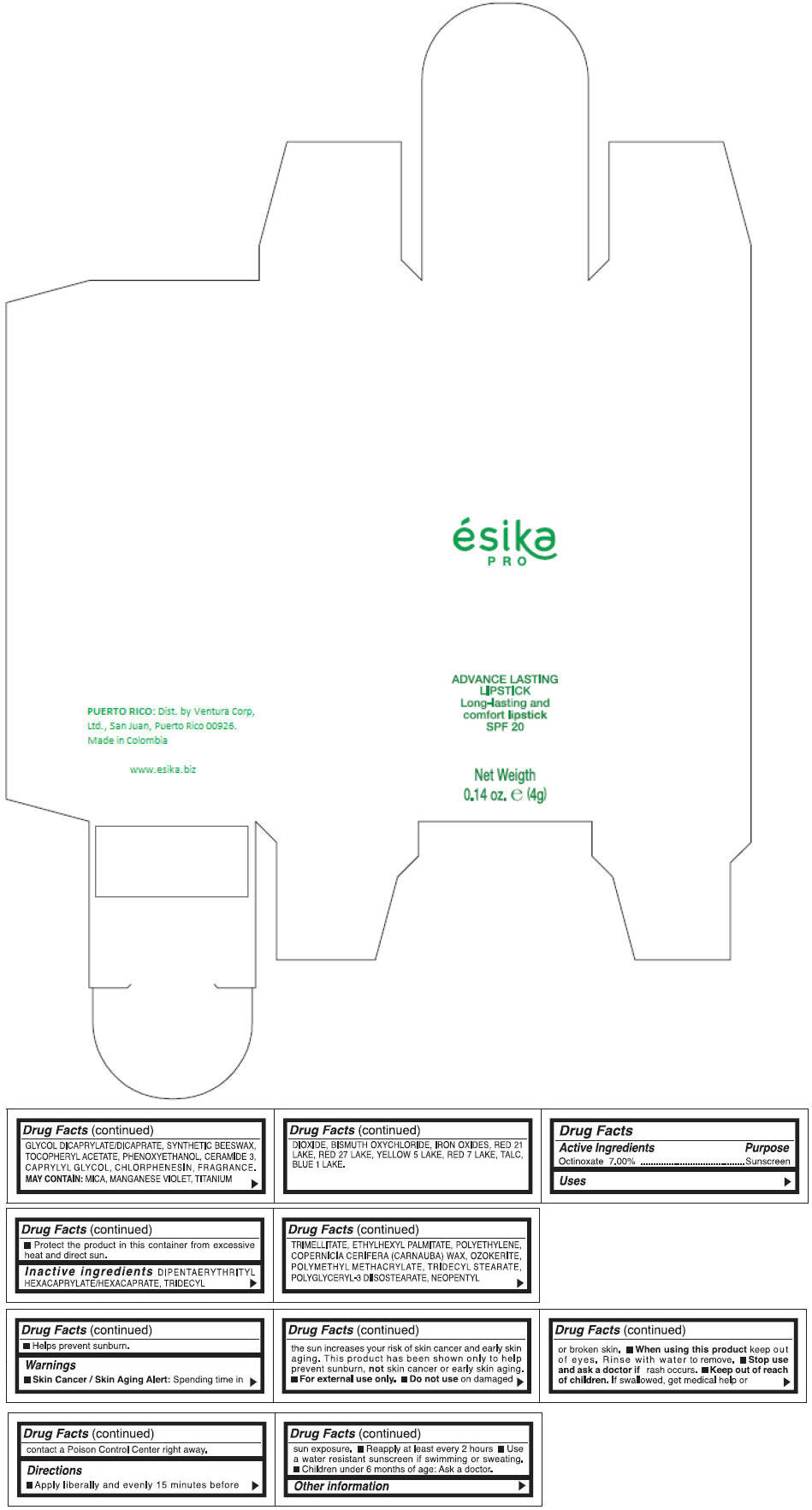
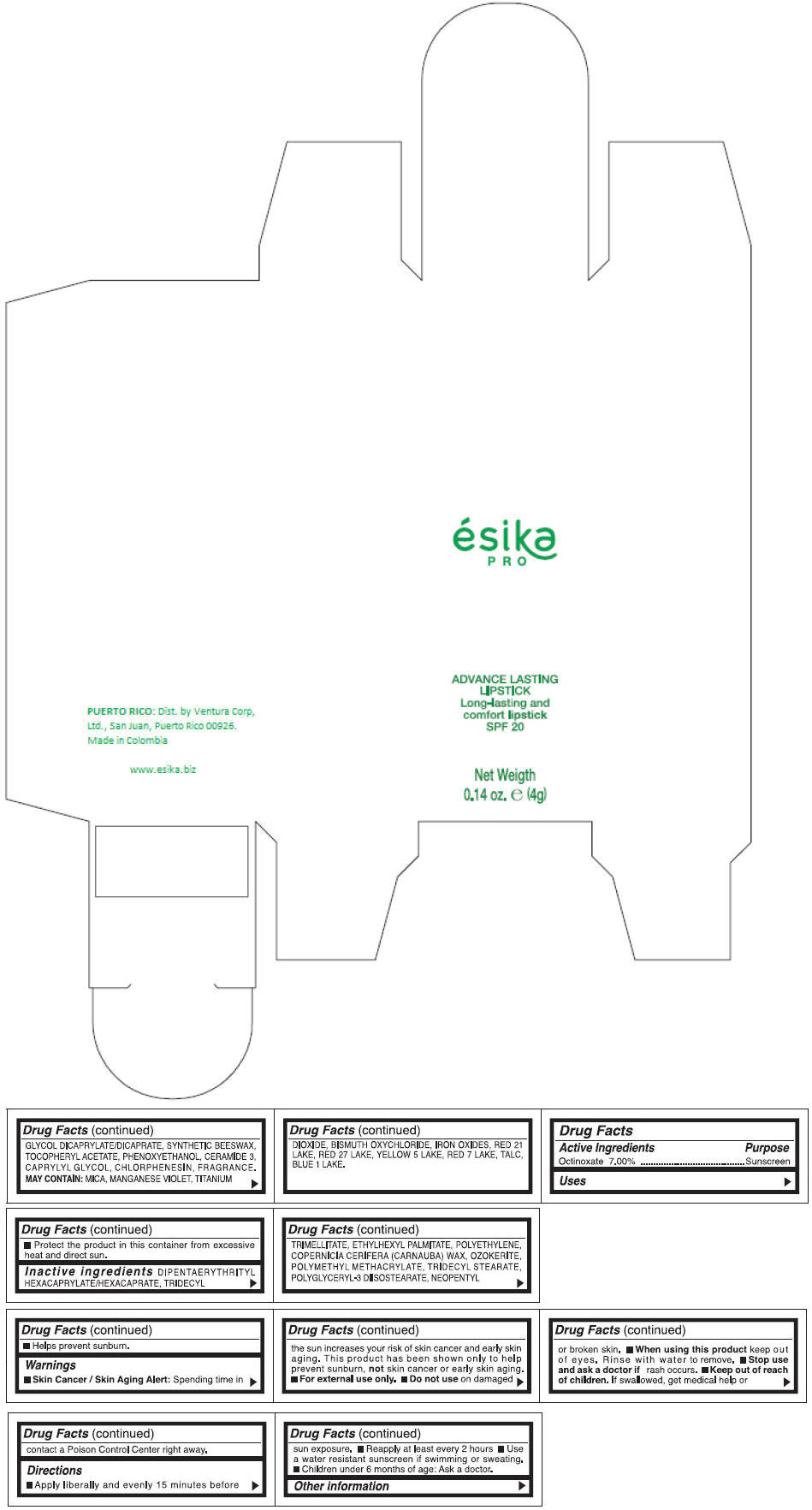
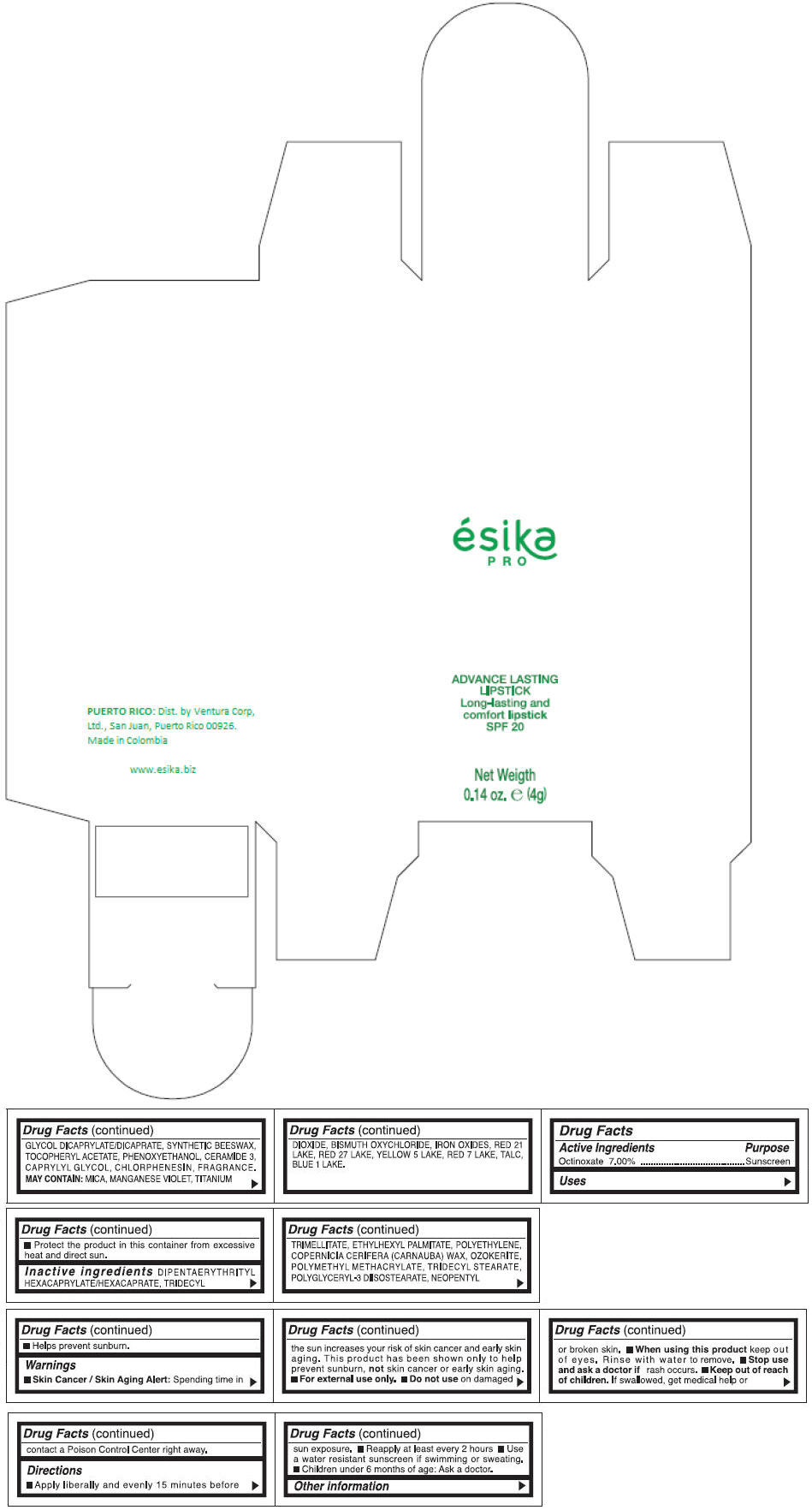
Inactive ingredients
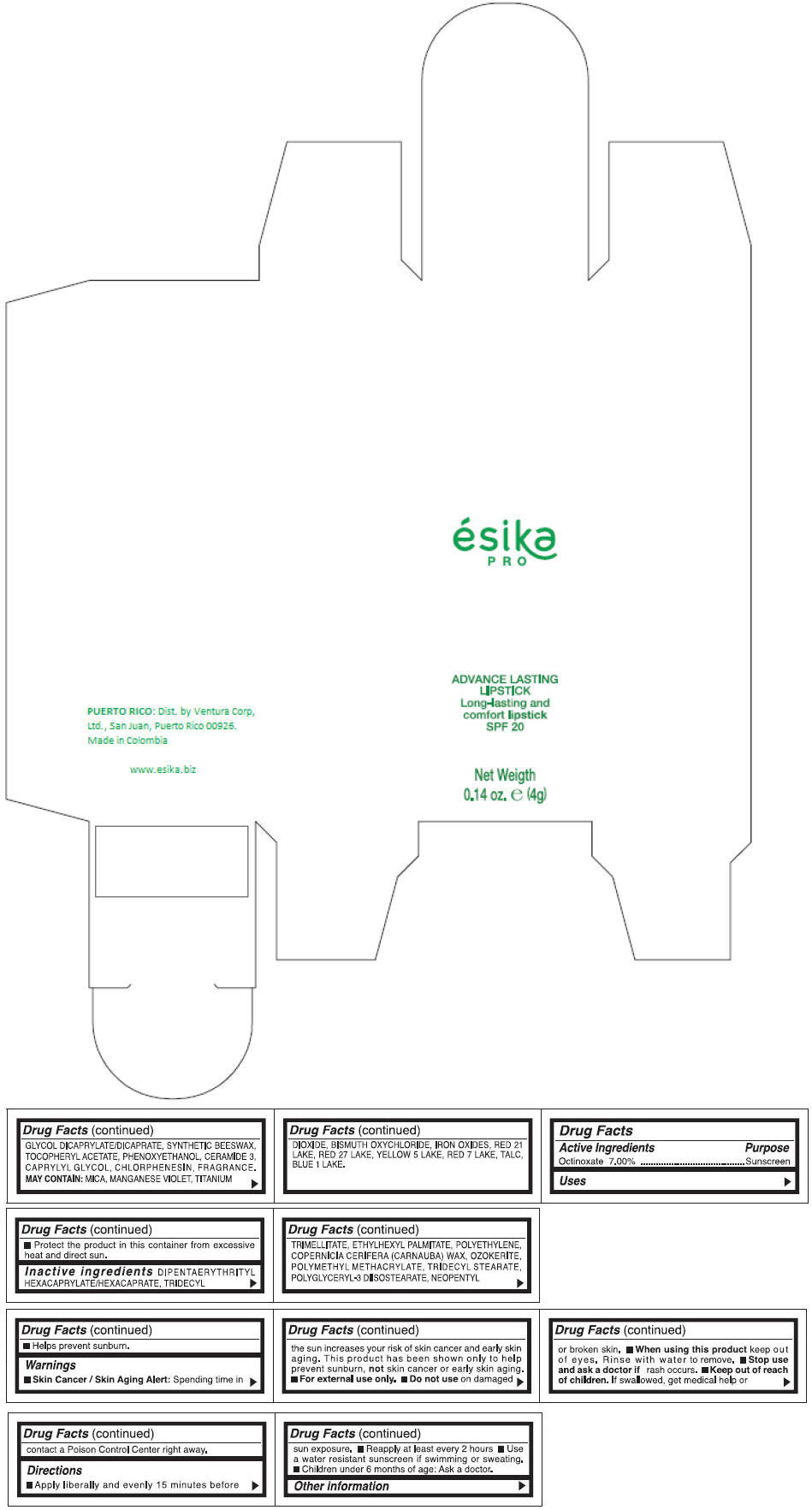
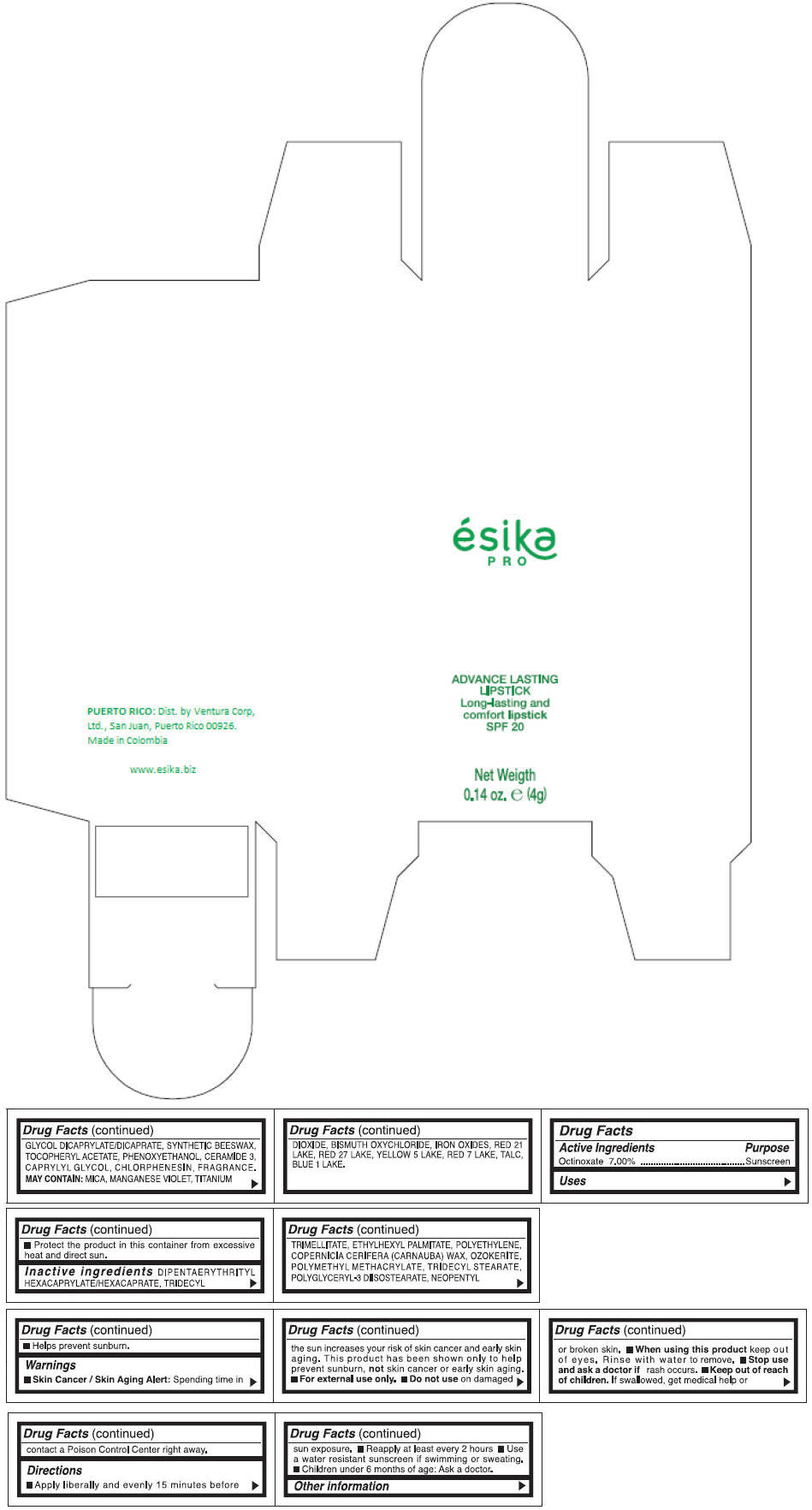
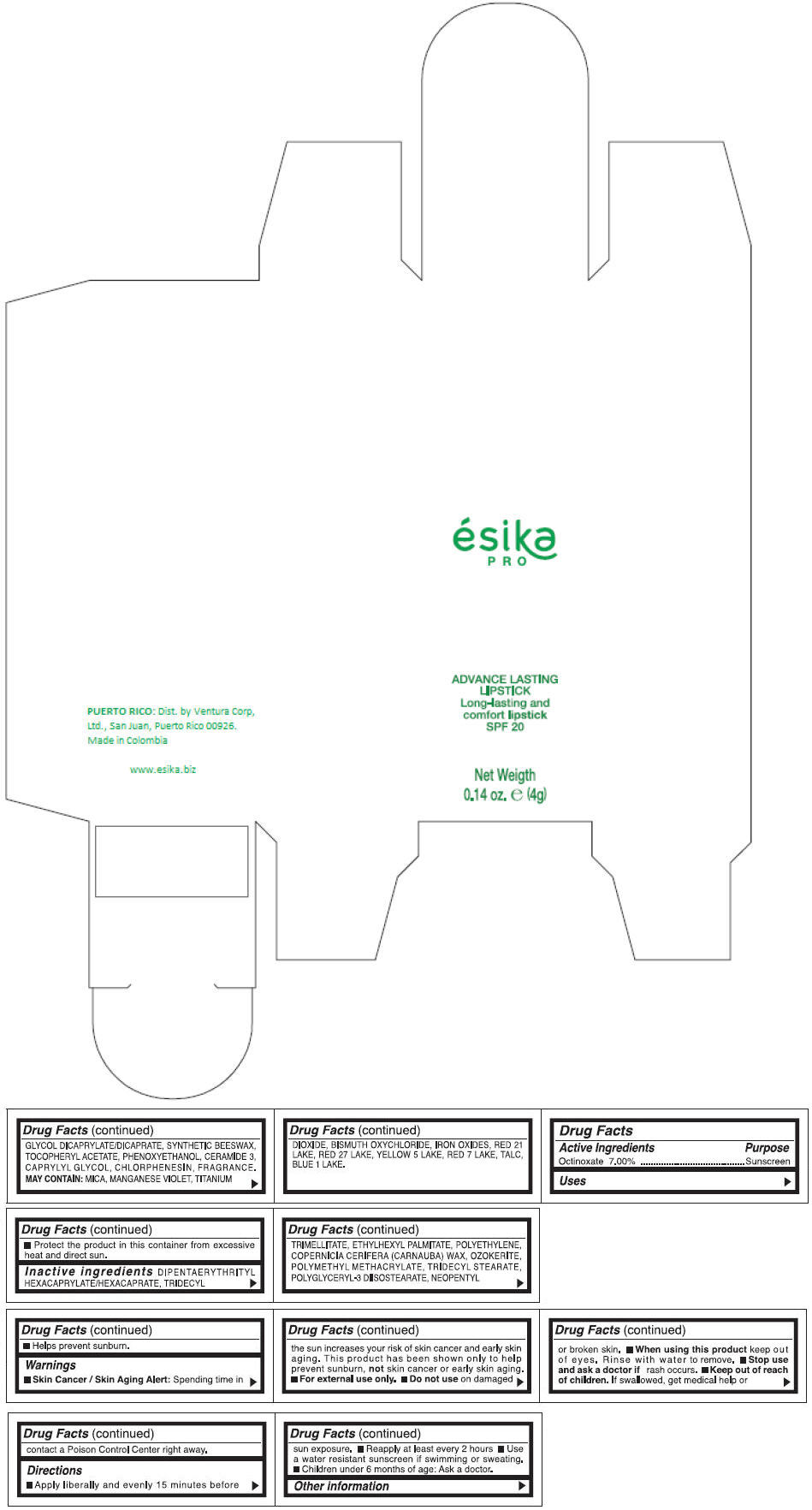
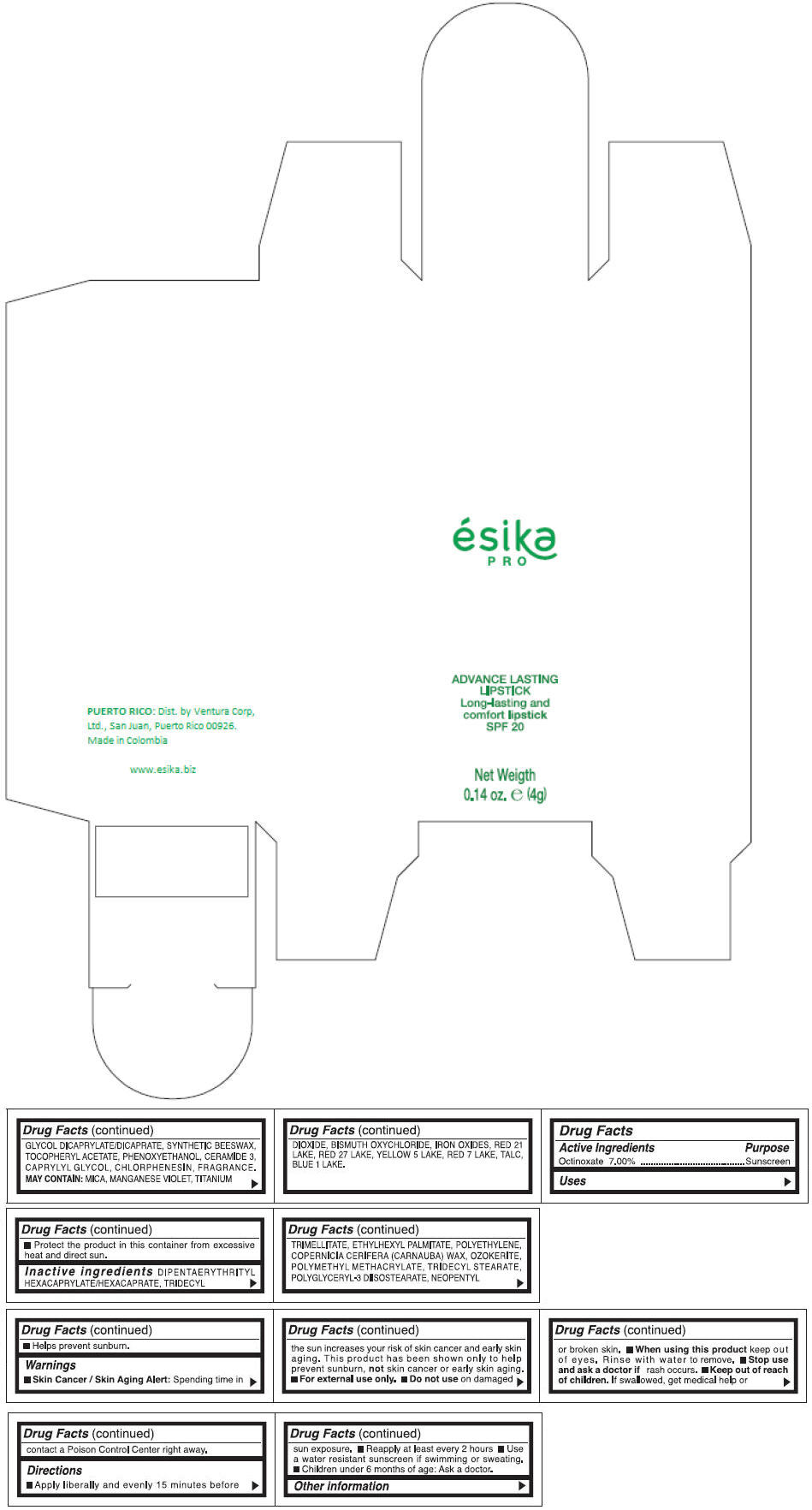
DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE, TRIDECYL TRIMELLITATE, ETHYLHEXYL PALMITATE, POLYETHYLENE, COPERNICIA CERIFERA (CARNAUBA) WAX, OZOKERITE, POLYMETHYL METHACRYLATE, TRIDECYL STEARATE, POLYGLYCERYL-3 DIISOSTEARATE, NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE, SYNTHETIC BEESWAX, TOCOPHERYL ACETATE, PHENOXYETHANOL, CERAMIDE 3, CAPRYLYL GLYCOL, CHLORPHENESIN, FRAGRANCE.
MAY CONTAIN :
MICA, MANGANESE VIOLET, TITANIUM DIOXIDE, BISMUTH OXYCHLORIDE, IRON OXIDES, RED 21 LAKE, RED 27 LAKE, YELLOW 5 LAKE, RED 7 LAKE, TALC, BLUE 1 LAKE.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (MARRON HAVANA) - BROWN
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (NATURAL DUNE) - PINK
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (MARRON FANATIC) - RED
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (ROJO FIESTA) - RED
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (MARRON BAMBU) - BROWN
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (FUCSIA DELIRIO) - PINK
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (ROJO PASION) - RED
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (FUCSIA SUBLIME) - PINK
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (ROSA FIORELLE) - PINK
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (NARANJA OBSESION) - ORANGE
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (BORGOÑA SWEET) - PINK
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (VINO DESEO) - PURPLE
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - (FUCSIA TENTACION) - PINK
- PRINCIPAL DISPLAY PANEL - Kit Carton
-
INGREDIENTS AND APPEARANCE
ESIKA PRO ADVANCE LASTING LONG-LASTING AND COMFORT SPF 20 (MARRON HAVANA) - BROWN
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 13537-638 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.07 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) CERAMIDE 3 (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) MICA (UNII: V8A1AW0880) MANGANESE VIOLET (UNII: 72M48QQV8Q) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 21 (UNII: 08744Z6JNY) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 13537-638-02 1 in 1 BOX 1 NDC: 13537-638-01 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 04/17/2015 ESIKA PRO ADVANCE LASTING LONG-LASTING AND COMFORT SPF 20 (NATURAL DUNE) - PINK
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 13537-639 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.07 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) CERAMIDE 3 (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) MICA (UNII: V8A1AW0880) MANGANESE VIOLET (UNII: 72M48QQV8Q) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 21 (UNII: 08744Z6JNY) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 13537-639-04 1 in 1 BOX 1 NDC: 13537-639-03 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 04/17/2015 ESIKA PRO ADVANCE LASTING LONG-LASTING AND COMFORT SPF 20 (MARRON FANATIC) - RED
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 13537-640 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.07 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) CERAMIDE 3 (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) MICA (UNII: V8A1AW0880) MANGANESE VIOLET (UNII: 72M48QQV8Q) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 21 (UNII: 08744Z6JNY) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 13537-640-06 1 in 1 BOX 1 NDC: 13537-640-05 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 04/17/2015 ESIKA PRO ADVANCE LASTING LONG-LASTING AND COMFORT SPF 20 (ROJO FIESTA) - RED
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 13537-642 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.07 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) CERAMIDE 3 (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) MICA (UNII: V8A1AW0880) MANGANESE VIOLET (UNII: 72M48QQV8Q) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 21 (UNII: 08744Z6JNY) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 13537-642-08 1 in 1 BOX 1 NDC: 13537-642-07 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 04/17/2015 ESIKA PRO ADVANCE LASTING LONG-LASTING AND COMFORT SPF 20 (MARRON BAMBU) - BROWN
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 13537-643 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.07 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) CERAMIDE 3 (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) MICA (UNII: V8A1AW0880) MANGANESE VIOLET (UNII: 72M48QQV8Q) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 21 (UNII: 08744Z6JNY) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 13537-643-10 1 in 1 BOX 1 NDC: 13537-643-09 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 04/17/2015 ESIKA PRO ADVANCE LASTING LONG-LASTING AND COMFORT SPF 20 (FUCSIA DELIRIO) - PINK
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 13537-644 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.07 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) CERAMIDE 3 (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) MICA (UNII: V8A1AW0880) MANGANESE VIOLET (UNII: 72M48QQV8Q) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 21 (UNII: 08744Z6JNY) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 13537-644-12 1 in 1 BOX 1 NDC: 13537-644-11 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 04/17/2015 ESIKA PRO ADVANCE LASTING LONG-LASTING AND COMFORT SPF 20 (ROJO PASION) - RED
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 13537-645 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.07 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) CERAMIDE 3 (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) MICA (UNII: V8A1AW0880) MANGANESE VIOLET (UNII: 72M48QQV8Q) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 21 (UNII: 08744Z6JNY) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 13537-645-14 1 in 1 BOX 1 NDC: 13537-645-13 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 04/17/2015 ESIKA PRO ADVANCE LASTING LONG-LASTING AND COMFORT SPF 20 (FUCSIA SUBLIME) - PINK
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 13537-646 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.07 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) CERAMIDE 3 (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) MICA (UNII: V8A1AW0880) MANGANESE VIOLET (UNII: 72M48QQV8Q) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 21 (UNII: 08744Z6JNY) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 13537-646-16 1 in 1 BOX 1 NDC: 13537-646-15 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 04/17/2015 ESIKA PRO ADVANCE LASTING LONG-LASTING AND COMFORT SPF 20 (ROSA FIORELLE) - PINK
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 13537-647 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.07 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) CERAMIDE 3 (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) MICA (UNII: V8A1AW0880) MANGANESE VIOLET (UNII: 72M48QQV8Q) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 21 (UNII: 08744Z6JNY) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 13537-647-18 1 in 1 BOX 1 NDC: 13537-647-17 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 04/17/2015 ESIKA PRO ADVANCE LASTING LONG-LASTING AND COMFORT SPF 20 (NARANJA OBSESION) - ORANGE
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 13537-648 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.07 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) CERAMIDE 3 (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) MICA (UNII: V8A1AW0880) MANGANESE VIOLET (UNII: 72M48QQV8Q) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 21 (UNII: 08744Z6JNY) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 13537-648-20 1 in 1 BOX 1 NDC: 13537-648-19 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 04/17/2015 ESIKA PRO ADVANCE LASTING LONG-LASTING AND COMFORT SPF 20 (BORGONA SWEET) - PINK
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 13537-649 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.07 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) CERAMIDE 3 (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) MICA (UNII: V8A1AW0880) MANGANESE VIOLET (UNII: 72M48QQV8Q) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 21 (UNII: 08744Z6JNY) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 13537-649-22 1 in 1 BOX 1 NDC: 13537-649-21 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 04/17/2015 ESIKA PRO ADVANCE LASTING LONG-LASTING AND COMFORT SPF 20 (VINO DESEO) - PURPLE
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 13537-650 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.07 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) CERAMIDE 3 (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) MICA (UNII: V8A1AW0880) MANGANESE VIOLET (UNII: 72M48QQV8Q) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 21 (UNII: 08744Z6JNY) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 13537-650-24 1 in 1 BOX 1 NDC: 13537-650-23 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 04/17/2015 ESIKA PRO ADVANCE LASTING LONG-LASTING AND COMFORT SPF 20 (FUCSIA TENTACION) - PINK
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 13537-651 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.07 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) CERAMIDE 3 (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) MICA (UNII: V8A1AW0880) MANGANESE VIOLET (UNII: 72M48QQV8Q) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 21 (UNII: 08744Z6JNY) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 13537-651-26 1 in 1 BOX 1 NDC: 13537-651-25 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 04/17/2015 ESIKA PRO ADVANCE LASTING LONG-LASTING AND COMFORT SPF 20
octinoxate kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 13537-688 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 13537-688-28 2 in 1 CARTON 1 NDC: 13537-688-27 1 in 1 TUBE Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 TUBE 6.8 g Part 2 1 TUBE 6.8 g Part 3 1 TUBE 6.8 g Part 4 1 TUBE 6.8 g Part 5 1 TUBE 6.8 g Part 6 1 TUBE 6.8 g Part 7 1 TUBE 6.8 g Part 8 1 TUBE 6.8 g Part 9 1 TUBE 6.8 g Part 10 1 TUBE 6.8 g Part 11 1 TUBE 6.8 g Part 12 1 TUBE 6.8 g Part 1 of 12 ESIKA PRO ADVANCE LASTING LONG-LASTING AND COMFORT SPF 20 (MARRON HAVANA) - BROWN
octinoxate lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.07 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) CERAMIDE 3 (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) MICA (UNII: V8A1AW0880) MANGANESE VIOLET (UNII: 72M48QQV8Q) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 21 (UNII: 08744Z6JNY) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 6.8 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 04/17/2015 Part 2 of 12 ESIKA PRO ADVANCE LASTING LONG-LASTING AND COMFORT SPF 20 (NATURAL DUNE) - PINK
octinoxate lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.07 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) CERAMIDE 3 (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) MICA (UNII: V8A1AW0880) MANGANESE VIOLET (UNII: 72M48QQV8Q) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 21 (UNII: 08744Z6JNY) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 6.8 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 04/17/2015 Part 3 of 12 ESIKA PRO ADVANCE LASTING LONG-LASTING AND COMFORT SPF 20 (MARRON FANATIC) - RED
octinoxate lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.07 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) CERAMIDE 3 (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) MICA (UNII: V8A1AW0880) MANGANESE VIOLET (UNII: 72M48QQV8Q) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 21 (UNII: 08744Z6JNY) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 6.8 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 04/17/2015 Part 4 of 12 ESIKA PRO ADVANCE LASTING LONG-LASTING AND COMFORT SPF 20 (ROJO FIESTA) - RED
octinoxate lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.07 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) CERAMIDE 3 (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) MICA (UNII: V8A1AW0880) MANGANESE VIOLET (UNII: 72M48QQV8Q) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 21 (UNII: 08744Z6JNY) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 6.8 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 04/17/2015 Part 5 of 12 ESIKA PRO ADVANCE LASTING LONG-LASTING AND COMFORT SPF 20 (MARRON BAMBU) - BROWN
octinoxate lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.07 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) CERAMIDE 3 (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) MICA (UNII: V8A1AW0880) MANGANESE VIOLET (UNII: 72M48QQV8Q) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 21 (UNII: 08744Z6JNY) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 6.8 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 04/17/2015 Part 6 of 12 ESIKA PRO ADVANCE LASTING LONG-LASTING AND COMFORT SPF 20 (FUCSIA DELIRIO) - PINK
octinoxate lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.07 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) CERAMIDE 3 (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) MICA (UNII: V8A1AW0880) MANGANESE VIOLET (UNII: 72M48QQV8Q) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 21 (UNII: 08744Z6JNY) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 6.8 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 04/17/2015 Part 7 of 12 ESIKA PRO ADVANCE LASTING LONG-LASTING AND COMFORT SPF 20 (ROJO PASION) - RED
octinoxate lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.07 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) CERAMIDE 3 (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) MICA (UNII: V8A1AW0880) MANGANESE VIOLET (UNII: 72M48QQV8Q) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 21 (UNII: 08744Z6JNY) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 6.8 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 04/17/2015 Part 8 of 12 ESIKA PRO ADVANCE LASTING LONG-LASTING AND COMFORT SPF 20 (FUCSIA SUBLIME) - PINK
octinoxate lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.07 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) CERAMIDE 3 (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) MICA (UNII: V8A1AW0880) MANGANESE VIOLET (UNII: 72M48QQV8Q) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 21 (UNII: 08744Z6JNY) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 6.8 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 04/17/2015 Part 9 of 12 ESIKA PRO ADVANCE LASTING LONG-LASTING AND COMFORT SPF 20 (ROSA FIORELLE) - PINK
octinoxate lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.07 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) CERAMIDE 3 (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) MICA (UNII: V8A1AW0880) MANGANESE VIOLET (UNII: 72M48QQV8Q) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 21 (UNII: 08744Z6JNY) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 6.8 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 04/17/2015 Part 10 of 12 ESIKA PRO ADVANCE LASTING LONG-LASTING AND COMFORT SPF 20 (BORGONA SWEET) - PINK
octinoxate lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.07 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) CERAMIDE 3 (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) MICA (UNII: V8A1AW0880) MANGANESE VIOLET (UNII: 72M48QQV8Q) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 21 (UNII: 08744Z6JNY) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 6.8 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 04/17/2015 Part 11 of 12 ESIKA PRO ADVANCE LASTING LONG-LASTING AND COMFORT SPF 20 (VINO DESEO) - PURPLE
octinoxate lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.07 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) CERAMIDE 3 (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) MICA (UNII: V8A1AW0880) MANGANESE VIOLET (UNII: 72M48QQV8Q) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 21 (UNII: 08744Z6JNY) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 6.8 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 04/17/2015 Part 12 of 12 ESIKA PRO ADVANCE LASTING LONG-LASTING AND COMFORT SPF 20 (FUCSIA TENTACION) - PINK
octinoxate lipstickProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 0.07 g in 1 g Inactive Ingredients Ingredient Name Strength DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) ETHYLHEXYL PALMITATE (UNII: 2865993309) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) TRIDECYL STEARATE (UNII: A8OE252M6L) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) CERAMIDE 3 (UNII: 4370DF050B) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) MICA (UNII: V8A1AW0880) MANGANESE VIOLET (UNII: 72M48QQV8Q) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) FERRIC OXIDE RED (UNII: 1K09F3G675) D&C RED NO. 21 (UNII: 08744Z6JNY) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 7 (UNII: ECW0LZ41X8) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 6.8 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 04/17/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 04/17/2015 Labeler - Ventura Corporation LTD (602751344) Establishment Name Address ID/FEI Business Operations Bel Star S.A. (Colombia) 880160197 MANUFACTURE(13537-638, 13537-639, 13537-640, 13537-642, 13537-643, 13537-644, 13537-645, 13537-646, 13537-647, 13537-648, 13537-649, 13537-650, 13537-651, 13537-688)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.