CBD CRYOTHERAPY PAIN RELIEF by Global Widget, LLC / Global Widget LLC Drug Facts
CBD CRYOTHERAPY PAIN RELIEF by
Drug Labeling and Warnings
CBD CRYOTHERAPY PAIN RELIEF by is a Otc medication manufactured, distributed, or labeled by Global Widget, LLC, Global Widget LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
CBD CRYOTHERAPY PAIN RELIEF- menthol gel
Global Widget, LLC
----------
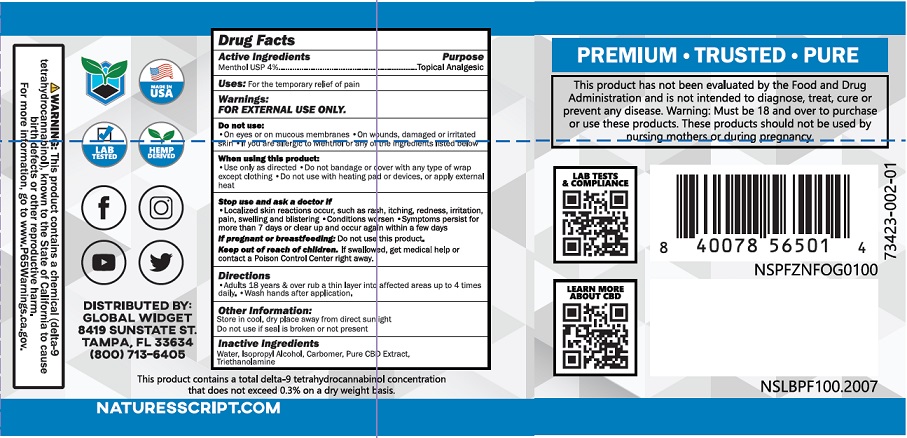
Drug Facts
Warnings
FOR EXTERNAL USE ONLY
Do not use:
- On eyes or on mucous membranes
- On wounds, damaged or irritated skin
- If you are allergic to Menthol or any of the ingredients listed below
When using this product:
- Use only as directed
- Do not bandage or cover with any type of wrap except clothing
- Do not use with heating pad or devices, or apply external heat
Directions
Adults 18 years & over rub a thin layer into affected areas up to 4 times daily.
Wash hands after application.
| CBD CRYOTHERAPY PAIN RELIEF
menthol gel |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Global Widget, LLC (089584863) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Global Widget, LLC | 089584863 | manufacture(73423-002) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Global Widget LLC | 118504011 | manufacture(73423-002) | |
Revised: 12/2023
Document Id: 0c49a83c-cdf7-3f85-e063-6294a90a34e1
Set id: e41e2811-f1c5-46f5-8124-5d66c3b10e6b
Version: 4
Effective Time: 20231211
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.