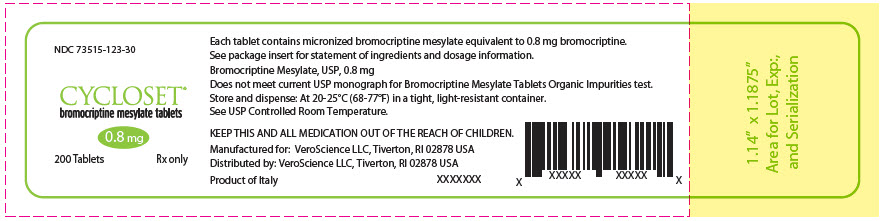
CYCLOSET- bromocriptine mesylate tablet
CYCLOSET by
Drug Labeling and Warnings
CYCLOSET by is a Prescription medication manufactured, distributed, or labeled by VEROSCIENCE LLC, Patheon Pharmaceuticals Inc., AMRI Italy S.r.l.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use CYCLOSET safely and effectively. See full prescribing information for CYCLOSET.
CYCLOSET® (bromocriptine mesylate tablets), for oral use
Initial U.S. Approval: 1978INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
- Taken within two hours after waking in the morning with food (2.1)
- Initial dose is one tablet (0.8 mg) daily increased weekly by one tablet until maximal tolerated daily dose of 1.6 to 4.8 mg is achieved. (2.2)
- Limit dose to 1.6 mg daily during concomitant use of a moderate CYP3A4 inhibitor. Avoid concomitant use with strong CYP3A4 inhibitors. (2.3)
DOSAGE FORMS AND STRENGTHS
Tablets: 0.8 mg (3)
CONTRAINDICATIONS
- Do not use in patients with hypersensitivity to ergot-related drugs, bromocriptine or to any of the excipients in CYCLOSET. (4)
- Do not use in patients with syncopal migraines. May precipitate hypotension. (4)
- Do not use in nursing women. May inhibit lactation. Postmarketing reports of stroke in this patient population. (4, 6.2, 8.3)
WARNINGS AND PRECAUTIONS
- Hypotension: Can cause orthostatic hypotension and syncope, particularly upon initiation or dose escalation. Use caution in patients taking antihypertensive medications. Assess orthostatic vital signs prior to initiation of CYCLOSET and periodically thereafter. Advise patients during early treatment to avoid situations that could lead to injury if syncope was to occur. (5.1, 6.1)
- Psychosis: May exacerbate psychotic disorders or reduce the effectiveness of drugs that treat psychosis. Use in patients with severe psychotic disorders is not recommended. (5.2)
- Somnolence: May cause somnolence. Advise patients not to operate heavy machinery if symptoms of somnolence occur. (5.3)
- Interaction with dopamine antagonists: Concomitant use with dopamine antagonists such as neuroleptic agents may diminish the effectiveness of both drugs. Concomitant use is not recommended. (5.4, 7)
- Other dopamine receptor agonists: Effectiveness and safety are unknown in patients already taking dopamine receptor agonists for other indications. Concomitant use is not recommended. (5.5)
- Macrovascular outcomes: There have been no clinical studies establishing conclusive evidence of macrovascular risk reduction with CYCLOSET or any other antidiabetic drug. CYCLOSET does not increase the risk of macrovascular events. (5.6, 6.1)
ADVERSE REACTIONS
In controlled clinical trials, adverse reactions reported in ≥5% of patients treated with CYCLOSET and reported more commonly than in patients treated with placebo, included nausea, fatigue, dizziness, vomiting, and headache. (6.1)
Postmarketing reports with higher doses of bromocriptine used for other indications include psychotic disorders, hallucinations, and fibrotic complications. (6.2)
To report SUSPECTED ADVERSE REACTIONS, contact VeroScience, LLC at 1-800-321-4576 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- May increase the unbound fraction of highly protein-bound therapies, altering their effectiveness and safety profiles. (7)
- May increase ergot-related side effects or reduce ergot effectiveness for migraines if co-administered within 6 hours of ergot-related drugs. (7)
- Extensively metabolized by CYP3A4. Limit CYCLOSET dose to 1.6 mg/day during concomitant use of moderate CYP3A4 inhibitors. Avoid concomitant use of CYCLOSET with strong CYP3A4 inhibitors. (2.3, 7)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 2/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Type 2 Diabetes Mellitus
1.2 Important Limitations of Use
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosing
2.2 Titration
2.3 Use with Concomitant Therapy
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypotension
5.2 Psychotic Disorders
5.3 Somnolence
5.4 Interaction with Dopamine Receptor Antagonists
5.5 Other Dopamine Receptor Agonists
5.6. Macrovascular Outcomes
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Monotherapy
14.2 Combination Therapy
14.3 Changes in Lipids and Blood Pressure
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosing
The recommended dose of CYCLOSET is 1.6 mg to 4.8 mg administered once daily within two hours after waking in the morning. CYCLOSET should be taken with food to potentially reduce gastrointestinal side effects such as nausea.
2.2 Titration
CYCLOSET should be initiated at one tablet (0.8 mg) and increased by one tablet per week until a maximum daily dose of 6 tablets (4.8 mg) or until the maximal tolerated number of tablets between 2 and 6 per day is reached.
2.3 Use with Concomitant Therapy
CYCLOSET dose should not exceed 1.6 mg once daily during concomitant use of a moderate CYP3A4 inhibitor (e.g., erythromycin). Avoid concomitant use of CYCLOSET and strong CYP3A4 inhibitors (e.g., azole antimycotics, HIV protease inhibitors) and ensure adequate washout of the strong CYP3A4 inhibitor drug before initiating CYCLOSET treatment [see Drug Interactions (7), Clinical Pharmacology (12.3)].
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
CYCLOSET is contraindicated in:
- Patients with known hypersensitivity to bromocriptine, ergot-related drugs, or any of the excipients in CYCLOSET.
- Patients with syncopal migraine. Bromocriptine increases the likelihood of a hypotensive episode among patients with syncopal migraine. Loss of consciousness during a migraine may reflect dopamine receptor hypersensitivity. CYCLOSET is a dopamine receptor agonist and may, therefore, potentiate the risk for syncope in these patients.
- Women who are nursing their children. CYCLOSET may inhibit lactation. There are postmarketing reports of stroke in this patient population although causality has not been proven [see Use in Specific Populations (8.3)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypotension
Hypotension, including orthostatic hypotension, can occur, particularly upon initiation of CYCLOSET therapy and with dose escalation. In a 52-week, randomized clinical trial of 3070 patients, hypotension was reported in 2.2% of patients randomized to CYCLOSET compared to 0.8% of patients randomized to placebo. Among CYCLOSET-treated patients reporting symptomatic hypotension, 98% were on at least one blood pressure medication compared to 73% on such medication in the total study population. In this trial, six CYCLOSET-treated patients (0.3%) reported an adverse event of orthostatic hypotension compared to 2 (0.2%) placebo-treated patients. All six patients were taking antihypertensive medications. Hypotension can result in syncope. In this trial, syncope due to any cause was reported in 1.6% of CYCLOSET-treated patients and 0.7% of placebo-treated patients [see Adverse Reactions (6.1)]. As a precaution, assessment of orthostatic vital signs is recommended prior to initiation of CYCLOSET and periodically thereafter. During early treatment with CYCLOSET, patients should be advised to make slow postural changes and to avoid situations that could lead to serious injury if syncope was to occur. Use caution in patients taking antihypertensive medications.
5.2 Psychotic Disorders
In patients with severe psychotic disorders, treatment with a dopamine receptor agonist such as CYCLOSET may exacerbate the disorder or may diminish the effectiveness of drugs used to treat the disorder. Therefore, the use of CYCLOSET in patients with severe psychotic disorders in not recommended.
5.3 Somnolence
CYCLOSET may cause somnolence. In a 52-week, randomized clinical trial, 4.3% of CYCLOSET-treated patients and 1.3% of placebo-treated patients reported somnolence as an adverse event. None of these events were reported as serious, and the majority of patients reported resolution of somnolence over time. Patients should be made aware of this potential side effect, particularly when initiating therapy with CYCLOSET. Patients experiencing somnolence should refrain from driving or operating heavy machinery.
5.4 Interaction with Dopamine Receptor Antagonists
Dopamine receptor antagonists, including neuroleptic agents that have dopamine D2 receptor antagonist properties (e.g., clozapine, olanzapine, ziprasidone), may reduce the effectiveness of CYCLOSET, and CYCLOSET may reduce the effectiveness of these agents. CYCLOSET has not been studied in patients taking neuroleptic drugs. The concomitant use of CYCLOSET and dopamine receptor antagonists, including neuroleptic drugs, is not recommended.
5.5 Other Dopamine Receptor Agonists
Other dopamine receptor agonists are indicated for the treatment of Parkinson's disease, hyperprolactinemia, restless leg syndrome, acromegaly, and other disorders. The effectiveness and safety of CYCLOSET in patients who are already taking one of these other dopamine receptor agonists is unknown. Concomitant use is not recommended.
5.6. Macrovascular Outcomes
There have been no clinical studies establishing conclusive evidence of macrovascular risk reduction with CYCLOSET or any other antidiabetic drug. In a 52-week, randomized clinical trial, CYCLOSET use was not associated with an increased risk for adverse cardiovascular events [see Adverse Reactions (6.1)].
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, the adverse reaction rates reported in one clinical trial may not be easily compared to those rates reported in another clinical trial, and may not reflect the rates actually observed in clinical practice.
In the pooled CYCLOSET Phase 3 clinical trials (CYCLOSET N = 2,298; placebo N = 1,266), adverse events leading to discontinuation occurred in 539 (24%) CYCLOSET-treated patients and 118 (9%) placebo-treated patients. This between-group difference was driven mostly by gastrointestinal adverse events, particularly nausea.
The CYCLOSET safety trial was a 52-week, placebo-controlled study that included patients treated only with diet therapy or with other antidiabetic medications. A total of 3,070 patients were randomized to CYCLOSET (titrated to 1.6 to 4.8 mg daily, as tolerated) or placebo. The study population had a mean baseline age of 60 years (range 27-80) and 33% were 65 years of age or older. Approximately 43% of the patients were female, 68% were Caucasian, 17% were Black, 13% were Hispanic, and 1% were Asian. The mean baseline body mass index was 32 kg/m2. The mean duration of diabetes at baseline was 8 years and the mean baseline HbA1c was 7.0% with a mean baseline fasting plasma glucose of 142 mg/dL. At baseline, 12% of patients were treated with diet only, 40% were treated with one oral antidiabetic agent, 33% were treated with two oral antidiabetic agents, and 16% were treated with insulin alone or insulin in combination with an oral antidiabetic agent. At baseline, 76% of patients reported a history of hypercholesterolemia, 75% reported a history of hypertension, 11% reported a history of revascularization surgery, 10% reported a history of myocardial infarction, 10% reported a history of angina, and 5% reported a history of stroke. Forty-seven percent of the CYCLOSET-treated patients and 32% of the placebo-treated patients prematurely discontinued treatment. Adverse events leading to discontinuation of study drug occurred among 24% of the CYCLOSET-treated patients and 15% of the placebo-treated patients. This between-group difference was driven mostly by gastrointestinal adverse events, particularly nausea.
Table 1 summarizes the adverse events reported in ≥5% of patients treated with CYCLOSET in the Phase 3 clinical trials regardless of investigator assessment of causality. The most commonly reported adverse events (nausea, fatigue, vomiting, headache, dizziness) lasted a median of 14 days and were more likely to occur during the initial titration of CYCLOSET. None of the reports of nausea or vomiting were described as serious. There were no differences in the pattern of common adverse events across race groups or age groups (<65 years old vs. >65 years old). In the 52-week CYCLOSET safety trial, 11.5% of CYCLOSET-treated women compared to 3.6% of placebo-treated women reported vomiting. In this same trial, 5.4% of CYCLOSET-treated men compared to 2.8% of placebo-treated men reported vomiting.
Table 1: Adverse Events Reported in Phase 3 Clinical Trials of CYCLOSET (≥5% of Patients and Numerically More Frequent in CYCLOSET-Treated Patients than in Placebo-Treated Patients, Regardless of Investigator Assessment of Causality*) Monotherapy CYCLOSET 1.6 mg – 4.8 mg
N (%)Placebo
N (%)- * All randomized subjects receiving at least one dose of study drug
- † The Safety Trial enrolled patients treated with diet or no more than 2 antidiabetic medications (metformin, insulin secretagogues such as a sulfonylurea, thiazolidinediones, alpha glucosidase inhibitors, and/or insulin).
N = 159 N = 80 N = 79 Nausea 26 (32.5) 6 (7.6) Rhinitis 11 (13.8) 3 (3.8) Headache 10 (12.5) 7 (8.9) Asthenia 10 (12.5) 5 (6.3) Dizziness 10 (12.5) 6 (7.6) Constipation 9 (11.3) 3 (3.8) Sinusitis 8 (10.0) 2 (2.5) Diarrhea 7 (8.8) 4 (5.1) Amblyopia 6 (7.5) 1 (1.3) Dyspepsia 6 (7.5) 2 (2.5) Vomiting 5 (6.3) 1 (1.3) Infection 5 (6.3) 4 (5.1) Anorexia 4 (5.0) 1 (1.3) Adjunct to Sulfonylurea (2 pooled 24-week studies) N = 494 N = 244 N = 250 Nausea 62 (25.4) 12 (4.8) Asthenia 46 (18.9) 20 (8.0) Headache 41 (16.8) 40 (16.0) Flu syndrome 23 (9.4) 19 (7.6) Constipation 24 (9.8) 11 (4.4) Cold 20 (8.2) 20 (8.0) Dizziness 29 (11.9) 14 (5.6) Rhinitis 26 (10.7) 12 (4.8) Sinusitis 18 (7.4) 16 (6.4) Somnolence 16 (6.6) 5 (2.0) Vomiting 13 (5.3) 8 (3.2) Amblyopia 13 (5.3) 6 (2.4) 52-Week Safety Trial† N = 3070 N = 2054 N = 1016 Nausea 661 (32.2) 77 (7.6) Dizziness 303 (14.8) 93 (9.2) Fatigue 285 (13.9) 68 (6.7) Headache 235 (11.4) 84 (8.3) Vomiting 167 (8.1) 32 (3.1) Diarrhea 167 (8.1) 81 (8.0) Constipation 119 (5.8) 52 (5.1) Hypoglycemia
In the monotherapy trial, hypoglycemia was reported in 2 CYCLOSET-treated patients (3.7%) and 1 placebo-treated patient (1.3%). In the add-on to sulfonylurea trials, the incidence of hypoglycemia was 8.6% among the CYCLOSET-treated patients and 5.2% among the placebo-treated patients. In the CYCLOSET safety trial, hypoglycemia was defined as any of the following: 1) symptoms suggestive of hypoglycemia that promptly resolved with appropriate intervention, 2) symptoms with a measured glucose <60 mg/dL or 3) measured glucose below 49 mg/dL regardless of symptoms. In the 52-week safety trial, the incidence of hypoglycemia was 6.9% among the CYCLOSET-treated patients and 5.3% among the placebo-treated patients. In the safety trial, severe hypoglycemia was defined as an inability to self-treat neurological symptoms consistent with hypoglycemia that occurred in the setting of a measured blood glucose <50 mg/dL (or evidence of prompt resolution of these symptoms with administration of oral carbohydrates, subcutaneous glucagon, or intravenous glucose if blood glucose was not measured). In this trial, severe hypoglycemia was reported among 0.5% of CYCLOSET-treated patients and 1% of placebo-treated patients.
Syncope
In combined Phase 2 and 3 clinical trials, syncope was reported in 1.4% of the 2,500 CYCLOSET-treated patients and 0.6% of the 1,454 placebo-treated patients. Among the 3,070 patients studied in the 52-week safety trial, 33 CYCLOSET-treated patients (1.6%) and 7 placebo-treated patients (0.7%) reported an adverse event of syncope. The cause of syncope is not known in all cases [see Warnings and Precautions (5.1)]. In this trial, electrocardiograms were not available at the time of these events, but an assessment of routine electrocardiograms obtained during the course of the trial did not identify arrhythmias or QTc interval prolongation among the CYCLOSET-treated patients reporting syncope.
Central Nervous System
In the 52-week safety trial, somnolence and hypoesthesia were the only adverse events within the nervous system organ class that were reported at a rate of <5% and ≥1% and that occurred at a numerically greater frequency among CYCLOSET-treated patients (CYCLOSET 4.3% vs. placebo 1.3% for somnolence; CYCLOSET 1.4% vs. placebo 1.1% for hypoesthesia).
Serious Adverse Events and Cardiovascular Safety
The primary endpoint of the 52-week safety trial was the occurrence of all serious adverse events. A secondary endpoint was the occurrence of the composite of myocardial infarction, stroke, coronary revascularization, hospitalization for angina, and hospitalization for congestive heart failure.
All serious adverse events and cardiovascular endpoints were adjudicated by an independent event adjudication committee. Serious adverse events occurred in 176/2054 (8.5%) CYCLOSET-treated patients and 98/1016 (9.6%) placebo-treated patients. The hazard ratio comparing CYCLOSET to placebo for the time to first occurrence of a serious adverse event was 1.02 (upper bound of one-sided 96% confidence interval, 1.27). None of the serious adverse events grouped by System-Organ-Class occurred more than 0.3 percentage points higher with CYCLOSET than with placebo. The composite cardiovascular endpoint occurred in 31 (1.5%) CYCLOSET-treated patients and 30 (3.0%) placebo-treated patients. The hazard ratio comparing CYCLOSET to placebo for the time-to-first occurrence of the prespecified composite cardiovascular endpoint was 0.58 (two-sided 95% confidence interval, 0.35 – 0.96). Therefore, the incidence of this composite endpoint was not increased with CYCLOSET relative to placebo.
6.2 Postmarketing Experience
The active agent in CYCLOSET (bromocriptine mesylate) has been used in other formulations and often multiple times per day to treat hyperprolactinemia, acromegaly, and Parkinson's disease. The following adverse reactions have been identified during post approval use of bromocriptine mesylate for these indications, generally at doses higher than those approved for the treatment of type 2 diabetes. Because these reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Hallucinations
Hallucinations and mental confusion including delusions have been reported with bromocriptine. To date, there have been no reported cases of hallucinations or delusions among CYCLOSET-treated patients (n = 2500) in combined Phase 2 and 3 clinical trials of CYCLOSET.
Fibrotic-Related Complications
Fibrotic complications, including cases of retroperitoneal fibrosis, pulmonary fibrosis, pleural effusion, pleural thickening, pericarditis and pericardial effusions have been reported. These complications do not always resolve when bromocriptine is discontinued. Among several studies investigating a possible relation between bromocriptine exposure and cardiac valvulopathy, some events of cardiac valvulopathy have been reported, but no definitive association between bromocriptine mesylate use and clinically significant (moderate to severe) cardiac valvulopathy could be concluded.
To date, there have been no reported cases of retroperitoneal fibrosis, pulmonary infiltrates, pleural effusion, pleural thickening, pericarditis or pericardial effusions among the CYCLOSET–treated patients (n = 2500) in combined Phase 2 and 3 controlled clinical trials of CYCLOSET. There was one unconfirmed case (0.04% event rate) of an adverse event of pulmonary fibrosis classified as non-serious in a CYCLOSET-treated patient.
No cases of cardiac valvulopathy have been reported in any of the clinical studies to date with CYCLOSET.
Psychotic and Psychiatric Disorders
Psychotic disorders have been reported with bromocriptine. Additionally, pathological gambling has been reported with bromocriptine used to treat patients with Parkinson's disease. To date, there have been no reported cases of psychoses or pathological gambling among the CYCLOSET-treated patients (N = 2500) in combined Phase 2 and 3 controlled clinical trials of CYCLOSET.
Stroke
The indication for use of bromocriptine for inhibition of postpartum lactation was withdrawn based on postmarketing reports of stroke. Causality of bromocriptine use and the occurrence of stroke in this patient population has not been proven. Based on the CYCLOSET clinical trials, there is no evidence of increased risk for stroke when CYCLOSET is used to treat type 2 diabetes.
Neuroleptic-Like Malignant Syndrome
A neuroleptic-like malignant syndrome (manifested by high fever and increase in creatinine phosphokinase) has been reported upon cessation of bromocriptine treatment in patients with advanced Parkinson's disease or patients with secondary Parkinsonism. To date, there have been no reported cases of neuroleptic-like malignant syndrome in combined Phase 2 and 3 controlled clinical trials of CYCLOSET, including the Safety Trial (N = 2500). In the CYCLOSET Safety Trial, there were no reports of neuroleptic-like malignant syndrome during the 30 days of follow-up after cessation of CYCLOSET (N = 2054).
-
7 DRUG INTERACTIONS
- The active ingredient in CYCLOSET (bromocriptine mesylate) is highly bound to serum proteins. Therefore, CYCLOSET may increase the unbound fraction of other concomitantly used highly protein-bound therapies (e.g., salicylates, sulfonamides, chloramphenicol and probenecid), which may alter their effectiveness and risk for side effects.
- CYCLOSET is a dopamine receptor agonist. Concomitant use of dopamine receptor antagonists, such as neuroleptics (e.g., phenothiazines, butyrophenones, thioxanthenes), or metoclopramide may diminish the effectiveness of CYCLOSET, and CYCLOSET may diminish the effectiveness of these other therapies. The concurrent use of CYCLOSET with these agents has not been studied in clinical trials and is not recommended [see Warnings and Precautions (5.4)].
- CYCLOSET in combination with ergot-related drugs may cause an increase in the occurrence of ergot-related side effects, such as nausea, vomiting, and fatigue, and may also reduce the effectiveness of these ergot therapies when used to treat migraine. The concurrent use of these ergot agents within 6 hours of CYCLOSET dosing is not recommended.
- CYCLOSET is extensively metabolized by the liver via CYP3A4. Therefore, potent inhibitors or inducers of CYP3A4 may increase or reduce the circulating levels of CYCLOSET, respectively. Use caution when co-administering drugs that are inhibitors or inducers of CYP3A4. CYCLOSET dose should not exceed 1.6 mg once daily during concomitant use of a moderate CYP3A4 inhibitor (e.g., erythromycin). Concomitant use of strong CYP3A4 inhibitors (e.g., azole antimycotics, HIV protease inhibitors) with CYCLOSET should be avoided. Ensure adequate washout of the strong CYP3A4 inhibitor drug before initiating CYCLOSET treatment [see Clinical Pharmacology (12.3)].
- There are postmarketing reports of hypertension and tachycardia when bromocriptine was co-administered with sympathomimetic drugs (e.g., phenylpropanolamine and isometheptene) in postpartum women. There are limited clinical trial data supporting the safety of co-administering sympathomimetic drugs and CYCLOSET for more than 10 days. Therefore, concomitant use of these agents with CYCLOSET for more than 10 days duration is not recommended. Also, there are limited clinical trial data supporting the safety of selective 5-hydroxytryptamine1B (5-HT1B) agonists (e.g., sumatriptan) used concurrently with CYCLOSET, and the concomitant use of these agents with CYCLOSET should be avoided.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B
Two strains of pregnant rats were dosed orally with 3, 10, and 30 mg/kg/day (up to 72 times the human 4.8 mg daily dose, based on mg/m2 comparison) from gestation day 6-15 and with a single dose of 10 mg/kg on gestation day 5. Implantation was inhibited at 10 and 30 mg/kg (24 and 72 times the human 4.8 mg daily dose, based on mg/m2 comparison). When rats were dosed with 3, 10, and 30 mg/kg/day from gestation day 8-15 there was an increase in resorptions at 10 and 30 mg/kg. These effects were probably due to the dependence of implantation and the maintenance of gestation on prolactin in the rat and are not relevant for humans in which these events are not dependent on prolactin but on luteinizing hormone. There was no evidence of teratogenic effects in the rat.
In a small study in macaque monkeys given oral doses of 2 mg/kg/day (10 times the human 4.8 mg daily dose, based on mg/m2 comparison) during organogenesis no embryotoxic or teratologic effects were observed.
When male rats given oral doses of 2, 10, or 50 mg/kg/day (up to 120 times the human 4.8 mg daily dose, based on mg/m2 comparison) were mated with untreated females, there was a slight increase in pup loss in the 10 and 50 mg/kg/day groups (24-120 times the human 4.8 mg daily dose, based on mg/m2 comparison).
In two strains of pregnant rabbits treated from gestation day 6-18 with oral doses of 3, 10, 30, 100, and 300 mg/kg/day (up to 1400 times the human 4.8 mg daily dose, based on mg/m2 comparison) there was maternal toxicity and embryolethality at doses ≥10 mg/kg/day (48 times the human 4.8 mg daily dose, based on mg/m2 comparison). Low incidences of fetal abnormalities were observed at maternally toxic doses of 100-300 mg/kg/day (480-1400 times the human 4.8 mg daily dose, based on mg/m2 comparison). There were no treatment-related fetal abnormalities at doses ≤30 mg/kg/day (140 times the human 4.8 mg daily dose, based on mg/m2 comparison). Implantation was not affected in rabbits treated from gestation day 1-6 with oral doses of 100-300 mg/kg/day (480-1400 times the human 4.8 mg daily dose, based on mg/m2 comparison).
Studies in pregnant women have not shown that bromocriptine increases the risk of abnormalities when administered during pregnancy. Information concerning 1,276 pregnancies in women taking bromocriptine has been collected. In the majority of cases, bromocriptine was discontinued within the first 8 weeks of pregnancy (mean 29 days); however, 8 patients received the drug continuously throughout pregnancy. The mean daily dose for all patients was 5.8 mg (range 1-40 mg). Of these 1,276 pregnancies, there were 1,088 full-term deliveries (4 stillborn), 145 spontaneous abortions (11.4%), and 28 induced abortions (2.2%). Twelve extrauterine gravidities and 3 hydatidiform moles (twice in the same patient) caused early termination of pregnancy. These data compare favorably with the abortion rate (11-25%) cited for pregnancies induced by clomiphene citrate, menopausal gonadotropin, and chorionic gonadotropin. Although spontaneous abortions often go unreported, especially prior to 20 weeks of gestation, their frequency has been estimated to be 10-15% in the general population. The incidence of birth defects in the general population ranges from 2-4.5%. The incidence of birth defects in 1,109 live births from patients receiving bromocriptine was 3.3%. There is no suggestion that bromocriptine contributed to the type or incidence of birth defects in this group of infants.
A review of 4 different multicenter surveillance programs analyzed 2,351 pregnancies of 2,185 women treated with bromocriptine. In 583 children born of these women and followed for a minimum of 3-12 months, there was no suggestion of any adverse effect of intra-uterine exposure to bromocriptine on postnatal development. Most (≥75%) women had taken bromocriptine for 2-8 weeks and at 5-10 mg per day. Among 86 women having 93 pregnancies and treated with bromocriptine throughout pregnancy or from week 30 of pregnancy onwards (mostly for treatment of prolactinoma), there was only 1 spontaneous abortion. Similar results have been obtained in a Japanese hospital survey of 442 children born to 434 patients treated with bromocriptine during pregnancy and followed for at least one year.
Because the studies in humans cannot rule out the possibility of harm, CYCLOSET should be used during pregnancy only if clearly needed.
8.3 Nursing Mothers
CYCLOSET is contraindicated in women who are nursing their children. CYCLOSET contains bromocriptine which inhibits lactation. The indication for use of bromocriptine for inhibition of postpartum lactation was withdrawn based on postmarketing reports of stroke in this setting [see Contraindications (4), Adverse Reactions (6.2)].
8.4 Pediatric Use
The safety and effectiveness of CYCLOSET in pediatric patients have not been established.
8.5 Geriatric Use
In the two clinical trials of CYCLOSET add-on to sulfonylurea therapy and in the monotherapy trial, a total of 54 patients randomized to CYCLOSET were ≥65 years old. In the 52-week safety trial, 601 of the 2,054 CYCLOSET-treated patients (29%) were ≥65 years old. No overall differences in safety or effectiveness were observed between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out. [See Clinical Studies (14).]
-
10 OVERDOSAGE
With another formulation of bromocriptine mesylate, the most commonly reported signs and symptoms associated with acute overdose were nausea, vomiting, constipation, diaphoresis, dizziness, pallor, severe hypotension, malaise, confusion, lethargy, drowsiness, delusions, hallucinations, and repetitive yawning. The lethal dose has not been established.
Treatment of overdose consists of removal of the drug by emesis (if conscious), gastric lavage, activated charcoal, or saline catharsis. Careful supervision and recording of fluid intake and output is essential. Hypotension should be treated by placing the patient in the Trendelenburg position and administering intravenous fluids. If satisfactory relief of hypotension cannot be achieved by using the above measures to their fullest extent, vasopressors should be considered.
-
11 DESCRIPTION
CYCLOSET Tablets contain micronized bromocriptine mesylate, a dopamine receptor agonist. Bromocriptine mesylate is chemically designated [Ergotaman-3',6',18-trione, 2-bromo-12'-hydroxy-2'-(1-methylethyl)-5'-(2-methylpropyl)-, monomethanesulfonate (salt), (5'α)-]. CYCLOSET is a single enantiomer with absolute configuration 5R, 8R, 2'R, 5'S, 11'S, 12'S.
The structural formula of bromocriptine is shown below:
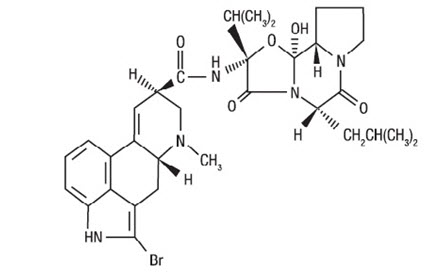
Bromocriptine mesylate in CYCLOSET is a white or slightly colored micronized crystalline powder with a molecular formula of C32H40BrN5O5∙CH4SO3 and a molecular weight of 750.72. CYCLOSET Tablets contain bromocriptine mesylate USP in an amount equivalent to 0.8 mg. of bromocriptine. Each tablet contains the following inactive ingredients: lactose, corn starch, magnesium stearate, colloidal silicon dioxide, and citric acid.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
CYCLOSET contains bromocriptine mesylate, a sympatholytic, dopamine D2 receptor agonist. In patients with type 2 diabetes, timed morning administration of CYCLOSET is associated with increased insulin sensitivity and glucose disposal and reduced fasting and postprandial hyperglycemia throughout the meals of the day without raising plasma insulin levels.
12.2 Pharmacodynamics
Postprandial Glucose and Insulin Response to a Meal
Patients with type 2 diabetes and inadequate glycemic control on diet alone were randomized to CYCLOSET or placebo in a 24-week monotherapy clinical trial. At baseline and study end, plasma samples for insulin and glucose were obtained before and 1 hour, and 2 hours after standardized meals for breakfast, lunch, and dinner. In this trial, once-daily (8 a.m.) CYCLOSET improved postprandial glucose without increasing plasma insulin concentrations.
Insulin-Mediated Glucose Disposal
Patients with type 2 diabetes and inadequate glycemic control on sulfonylurea therapy were randomized to CYCLOSET or placebo in a 16-week clinical trial. In this trial CYCLOSET therapy improved insulin-mediated glucose disposal and glucose tolerance and resulted in lower plasma glucose and HbA1c levels.
12.3 Pharmacokinetics
Absorption and Bioavailability
When administered orally, approximately 65-95% of the CYCLOSET dose of bromocriptine mesylate is absorbed. Due to extensive first-pass metabolism, approximately 7% of the dose reaches the systemic circulation. Under fasting conditions the time to maximum plasma concentration is 53 minutes. In contrast, following a standard high-fat meal, the time to maximum plasma concentration is increased to approximately 90-120 minutes. Also, the relative bioavailability of CYCLOSET is increased under fed as compared to fasting conditions by an average of approximately 55-65% (increase in AUCinf).
Distribution
Bromocriptine is 90-96% bound to plasma proteins. The volume of distribution is approximately 61 L.
Metabolism
Bromocriptine mesylate is extensively metabolized in the gastrointestinal tract and liver. Metabolism by CYP3A4 is the major metabolic pathway. Most of the absorbed dose (approximately 93%) undergoes first-pass metabolism. The remaining 7% reaches the systemic circulation.
Excretion
The major route of excretion of bromocriptine is in the bile with the remaining approximately 2-6% of an oral dose excreted via the urine. The elimination half-life is approximately 6 hours. Prior consumption of a standard high-fat meal has little to no effect on the elimination half-life of CYCLOSET.
Specific Populations
Renal Impairment
No pharmacokinetic studies have been conducted in patients with renal impairment. Although the kidney is a minor pathway for elimination of CYCLOSET, caution should be used in patients with renal impairment.
Hepatic Impairment
No pharmacokinetic studies have been conducted in patients with hepatic impairment. Because CYCLOSET is predominantly metabolized by the liver, caution should be used in patients with hepatic impairment.
Drug Interactions
In Vitro Assessment
Although bromocriptine is a competitive inhibitor of CYP3A4, in vivo drug interaction potential is low because the inhibitory potency for CYP3A4 is approximately 10,000-fold higher than the maximum plasma levels reached in vivo (Cmax of approximately 80-125 pg/mL) following a 4.8 mg oral dose of CYCLOSET.
Agents inducing CYP3A4 activity such as rifampin or dexamethasone would be expected to decrease CYCLOSET plasma levels. There was no significant in vitro inhibition of other major CYP450 enzymes (1A2, 2C9/19, 2D6) by bromocriptine.
In Vivo Assessment
The concomitant use of macrolide antibiotics such as erythromycin (250 mg four times a day), a known inhibitor of CYP3A4, along with bromocriptine (5 mg) was shown to increase the AUC (2.8-fold) and Cmax (4.6-fold) of bromocriptine [see Dosage and Administration (2.3), Drug Interactions (7)].
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
In a 74-week dietary study in mice at doses up to 50 mg/kg/day (56 times the human 4.8 mg daily dose, based on mg/m2 comparison), there was no evidence of tumorigenicity.
In a 100-week dietary carcinogenicity study in rats at doses of 1.8, 9.9 and 44.5 mg/kg/day (up to 106 times the human 4.8 mg daily dose, based on mg/m2 comparison), there was a significant increase in the incidence of malignant uterine neoplasms in the mid- and high-dose groups (24-106 times the human 4.8 mg daily dose, based on mg/m2 comparison). The increase in uterine neoplasms was probably due to the inhibition of prolactin-stimulated progesterone secretion resulting in estrogen domination and endometrial stimulation in the aging rat. Because prolactin does not play a role in human progesterone production this finding is unlikely to be clinically relevant.
Mutagenicity
Bromocriptine was not mutagenic in the in vitro Ames bacterial mutation assay, the V79 Chinese hamster fibroblast mutagenicity test, the in vivo bone marrow micronucleus test in mice and the in vivo Chinese hamster bone marrow chromosomal aberration test.
Impairment of Fertility
In female rats treated with oral doses of 1 and 3 mg/kg (2 to 7 times the human 4.8 mg daily dose, based on mg/m2 comparison) from 2 weeks prior to mating through 2 weeks post mating or throughout lactation, there was no effect on fertility. Postnatal pup weight gain was reduced dose-dependently in treated groups probably due to lactation inhibition.
In male rats treated with oral doses of 2, 10, and 50 mg/kg/day (up to 120 times the human 4.8 mg daily dose, based on mg/m2 comparison), there was no effect on mating or fertility.
-
14 CLINICAL STUDIES
A total of 3,723 patients with type 2 diabetes were randomized across 4 double-blind, placebo-controlled clinical trials conducted to evaluate the safety and glycemic efficacy of CYCLOSET. In the pooled 24-week monotherapy trial and the two 24-week add-on to sulfonylurea trials (N = 653), the mean age of the CYCLOSET-treated patients (N = 324) was 55 years, 71% were male and 73% Caucasian. In the 52-week safety trial (N = 3,070), the mean age for the entire study population was 60 years and 43% of patients were female, 68% were Caucasian, 17% were Black, 13% were Hispanic, and 1% were Asian.
In all 4 clinical trials, patients assigned to treatment with CYCLOSET received an initial dose of 0.8 mg, which was increased by 0.8 mg each week for 6 weeks (4.8 mg/day final dose) if no intolerance occurred or until the maximum tolerated dose ≥1.6 mg/day was reached. In patients with type 2 diabetes, treatment with CYCLOSET produced clinically significant improvements in HbA1c and postprandial glucose (PPG).
14.1 Monotherapy
A total of 159 overweight (body mass index ≥26.0 kg/m2 for males and ≥28.0 kg/m2 for females) adults with type 2 diabetes and inadequate glycemic control (HbA1c 7.5-11%) participated in a 24-week, placebo-controlled, monotherapy trial that evaluated the efficacy and safety of CYCLOSET as an adjunct to diet and exercise. Mean body weight at baseline was 93 kg in the CYCLOSET group and 96 kg in the placebo group. Mean HbA1c at baseline was 9.0% in the CYCLOSET group and 8.8% in the placebo group. Mean duration of diabetes at baseline was 5 years in the CYCLOSET group and 4 years in the placebo group. Of the 80 patients in the CYCLOSET group, 69% (N = 55) achieved the maximum daily dose of 4.8 mg. CYCLOSET improved HbA1c and fasting plasma glucose compared to placebo (Table 2). Mean change from baseline in body weight was +0.2 kg in the CYCLOSET group (N = 78) and +0.5 kg in the placebo group (N = 77).
Table 2: Changes in Glycemic Parameters in a 24-Week Placebo-Controlled Study of CYCLOSET as Monotherapy in Patients with Type 2 Diabetes* CYCLOSET
N = 80
(1.6 - 4.8 mg)Placebo
N = 79P-value calculated by ANOVA; *p = 0.05, **p = 0.005 - * intent-to-treat population with last observation carried forward
HbA1c (%) N = 74 N = 74 Baseline (mean) 9.0 8.8 Change from baseline (adj. mean) -0.1 0.3 Difference from placebo (adj. mean) -0.4* Fasting Plasma Glucose (mg/dL) N = 76 N = 75 Baseline (mean) 215 205 Change from baseline (adj. mean) 0 23 Difference from placebo (adj. mean) -23** 14.2 Combination Therapy
CYCLOSET Add-on to Sulfonylurea Therapy
Patients with type 2 diabetes and inadequate glycemic control (HbA1c 7.8-12.5%) on sulfonylurea therapy (mean HbA1c 9.4%) participated in Study L, a 24-week, randomized, double-blind, placebo-controlled trial that evaluated the safety and glycemic efficacy of CYCLOSET when added to stable sulfonylurea therapy. The mean duration of diabetes was 6 years in the CYCLOSET group and 8 years in the placebo group. The range of body mass index was 26-40 kg/m2 for men and 28-40 kg/m2 for women, with a mean of 32 kg/m2 in both treatment groups. Of the 122 patients in the CYCLOSET group, 83 (68%) achieved the maximum dose of study drug. The mean change from baseline in body weight was +0.9 kg in the CYCLOSET group and +0.5 kg in the placebo group.
In another similarly designed trial, Study K, patients with type 2 diabetes and inadequate glycemic control (HbA1c 7.8-12.5 %) on stable sulfonylurea therapy were randomized to add-on therapy with either CYCLOSET (N = 122) or placebo (N = 123). The range of body mass index was 26-40 kg/m2 for men and 28-40 kg/m2 for women, with a mean of 32 kg/m2 in the CYCLOSET group and 33 kg/m2 in the placebo group. Of the 122 patients in the CYCLOSET group, 91 (75%) achieved the maximum dose of study drug. Mean change from baseline in body weight was +1.4 kg in the CYCLOSET group and +0.5 kg in the placebo group. CYCLOSET improved HbA1c and fasting blood glucose concentrations compared to placebo (Table 3).
Table 3: Changes in Glycemic Parameters for CYCLOSET Versus Placebo in Two Add-on to Sulfonylurea Trials Study K* Study L* CYCLOSET Add-on to Sulfonylurea
N = 122Placebo Add-on to Sulfonylurea
N = 123CYCLOSET Add-on to Sulfonylurea
N = 122Placebo Add-on to Sulfonylurea
N = 127P-value calculated by ANOVA; *p≤ 0.001,**p = 0.02; ‡ p = 0.006 - * intent-to-treat population using last observation carried forward between-group change from baseline in HbA1c
HbA1c (%) n = 114 n = 122 n = 114 n = 123 Baseline (mean) 9.3 9.4 9.3 9.4 Change from baseline (adj. mean) -0.1 0.4 -0.4 0.3 Difference from placebo (adj. mean) -0.5* -0.6* Fasting Plasma Glucose (mg/dL) n = 116 n = 119 n = 113 n = 123 Baseline (mean) 216 227 220 226 Change from baseline (adj. mean) 10 28 3 23 Difference from placebo (adj. mean) -18** -20‡ CYCLOSET Add-on to Various Oral Antidiabetic Agents
Patients with type 2 diabetes receiving various antidiabetic therapies (mean baseline HbA1c 8.3%) participated in a 52-week randomized, double-blind, placebo-controlled safety trial [see Adverse Reactions (6.1)]. The daily CYCLOSET dose was initiated at 0.8 mg and increased by 0.8 mg each week for 6 weeks if no intolerance occurred or until the maximum tolerated dose ≥1.6 mg/day was reached. Approximately 70% of patients assigned to treatment with CYCLOSET reached the maximum daily dose of 4.8 mg. Physicians were instructed to adjust the dosage of concomitant diabetes therapies to avert hypoglycemia or uncontrolled hyperglycemia. Doses of background antidiabetic medications could be adjusted at any time during the trial and additional antidiabetic medications were permitted after week 12, if needed to maintain ideal glycemic control. Mean baseline HbA1c was 7.0% in both treatment groups. The least-squares mean change in HbA1c from baseline to week 24 was 0.0% with CYCLOSET (N = 2049) and +0.2% with placebo (N = 1015). Because many patients (60%) were already at treatment goal at baseline (HbA1c <7%), pre-specified subgroup analyses of glycemic efficacy (change in HbA1c from baseline to week 24) were conducted for patients who had inadequate glycemic control (baseline HbA1c ≥7.5%) on 1-2 oral antidiabetic therapies at the time of study entry. Patients receiving CYCLOSET, compared to placebo, experienced a significant improvement in HbA1c when used as adjunctive therapy to 1-2 oral antidiabetic medications, including the subgroup of patients treated only with background metformin + sulfonylurea (Table 4). The mean change in body weight for the glycemic efficacy subgroup (N = 559) from baseline to week 24 was -0.1 kg with CYCLOSET and +0.1 kg. The mean change in body weight for the entire study population (N = 3070) from baseline to week 52 was +0.2 kg with CYCLOSET and +0.1 kg with placebo.
Table 4: Changes in HbA1c from Baseline to Week 24 in the CYCLOSET Safety Trial Subgroup of Patients with Type 2 Diabetes and Inadequate Glycemic Control (Baseline HbA1c ≥7.5%) on 1-2 Oral Antidiabetic Medications* 24-Week Intent-to-Treat CYCLOSET Placebo P-value is based on an ANCOVA model with treatment and center as fixed effects, and baseline HbA1c as covariates; *p<0.001 - * intent-to-treat population using last observation carried forward between-group change from baseline in HbA1c
- † patients in the "metformin + sulfonylurea only" subgroup are also counted in the "adjunct to 1-2 oral antidiabetic medications" subgroup
Adjunct to 1-2 Oral Antidiabetic Medications N = 376 N = 183 HbA1c (%) Baseline mean 8.3 8.4 Change from baseline (adjusted mean) -0.4 0.0 Difference from placebo (adjusted mean) -0.5* % Patients achieving HbA1c of ≤7.0 25 9 Adjunct to Metformin + Sulfonylurea Only† N = 177 N = 90 HbA1c (%) Baseline mean 8.3 8.3 Change from baseline (adjusted mean) -0.5 0.0 Difference from placebo (adjusted mean) -0.5* % Patients achieving HbA1c of ≤7.0 27 9 14.3 Changes in Lipids and Blood Pressure
CYCLOSET does not have an unfavorable effect on fasting plasma lipids.
CYCLOSET has not demonstrated an unfavorable hypertensive effect on blood pressure. Hypotension has been reported with use of CYCLOSET in clinical trials [see Warnings and Precautions (5.1)].
-
16 HOW SUPPLIED/STORAGE AND HANDLING
CYCLOSET 0.8 mg tablets are WHITE and round with "C" on one side and "9" on the other.
The tablets are supplied as follows:
NDC: 73515-123-30 unit-of-use bottles of 200
NDC: 73515-123-21 unit-of-use bottles of 21 (samples only). -
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Patients should be informed of the potential risks and benefits of CYCLOSET and of alternative therapies. Patients should also be informed about the importance of adherence to dietary instructions, regular physical activity, periodic blood glucose monitoring and HbA1c testing, recognition and management of hypoglycemia and hyperglycemia, and assessment for diabetes complications. During periods of stress such as fever, trauma, infection, or surgery, medication requirements may change and patients should be advised to seek medical advice promptly.
Patients should be advised that they may develop postural (orthostatic) hypotension with or without symptoms such as dizziness, nausea, and diaphoresis. Hypotension and syncope may occur more frequently during initial therapy or with an increase in dose at any time. During early treatment with CYCLOSET, patients should be advised to make slow postural changes and to avoid situations that could predispose to serious injury if syncope was to occur.
Patients should be advised that CYCLOSET may cause somnolence. Advise patients not to operate heavy machinery if symptoms of somnolence occur.
Women who are nursing their children should be advised to not take CYCLOSET.
Physicians should instruct their patients to read the Patient Package Insert before starting CYCLOSET therapy and to reread it each time the prescription is renewed. Patients should be instructed to inform their healthcare provider if they develop any unusual symptoms or if any known symptom persists or worsens.
-
SPL UNCLASSIFIED SECTION
Manufactured for:
VeroScience, LLC
Tiverton, RI 02878 USADistributed by:
VeroScience, LLC
Tiverton, RI 02878 USAPrinted in USA
For information for healthcare professionals, call 1-To Be Determined.
For patent information: http://veroscience.com/products/patents.html
Cycloset is a registered trademark of VeroScience LLC, Tiverton, RI 02878 USA.
-
Patient Information
CYCLOSET® [Si'klo'set] (bromocriptine mesylate)
TabletsRead the Patient Information before you start taking CYCLOSET and each time you get a refill. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or treatment.
What is CYCLOSET?
CYCLOSET is a prescription medicine used with diet and exercise to lower blood sugar in adults with type 2 diabetes. CYCLOSET may be taken alone or with other medicines that also help to control blood sugar.
CYCLOSET has not been studied in children.
Who should not take CYCLOSET?
Do not take CYCLOSET if you:
- are allergic to bromocriptine or any of the other ingredients in CYCLOSET
- take ergot medicines. Ask your healthcare provider for a list of these medicines, if you are not sure
- are breastfeeding
- have fainting (syncopal) migraine headaches
Talk to your healthcare provider before taking CYCLOSET if you have any of these conditions.
What should I tell my healthcare provider before taking CYCLOSET?
Before taking CYCLOSET, tell your healthcare provider about all of your medical conditions, including if you:
- have type 1 diabetes mellitus
- have diabetic ketoacidosis
- have ever passed out or fainted
- have migraine headaches
- have or have had low blood pressure (hypotension)
- have or have had a mental health condition, especially a psychotic disorder
- are pregnant or plan to become pregnant. It is not known if CYCLOSET will harm your unborn baby. Talk with your healthcare provider if you are pregnant or plan to become pregnant.
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. Especially tell your healthcare provider if you take medicines for:
- mental health conditions, especially anti-psychotic medicines
- migraine or other types of headaches
- type 2 diabetes
Ask your healthcare provider or pharmacist for a list of medicines taken for these conditions, if you are not sure.
CYCLOSET may affect the way other medicines work, and other medicines may affect how CYCLOSET works.
Know the medicines you take. Keep a list of your medicines and show it to your healthcare provider and pharmacist when you get a new medicine.
How should I take CYCLOSET?
- Take CYCLOSET exactly as your healthcare provider tells you to take it.
- Take CYCLOSET by mouth each day.
- Take CYCLOSET with food.
- Take CYCLOSET within 2 hours after waking in the morning.
- If you miss your morning dose, wait until the next morning to take your medication.
- Do not take a double dose of CYCLOSET.
- During periods of stress on the body, such as fever, trauma, infection, or surgery, your medication needs may change. Contact your healthcare provider right away.
- If you take too much CYCLOSET, call your healthcare provider or go to the nearest emergency department right away.
- While taking CYCLOSET:
- check your blood sugar as your healthcare provider tells you to
- stay on your prescribed diet and exercise program
- learn to prevent, recognize, and manage low blood sugar (hypoglycemia), high blood sugar (hyperglycemia), and complications of diabetes
- see your healthcare provider for regular blood tests, including your blood sugar levels and hemoglobin HbA1c
What are the possible side effects of CYCLOSET?
CYCLOSET may cause serious side effects, including:
- Low blood pressure
- Fainting
- Severe dizziness which can be caused by postural hypotension. This can happen when your blood pressure lowers rapidly after you stand up from a lying down position.
The most common side effects of CYCLOSET include:
- nausea
- headache
- fatigue (somnolence). If you have somnolence from CYCLOSET you should not drive or use other heavy machines until the somnolence is better.
- dizziness
- vomiting
- low blood sugar (hypoglycemia), especially when used with another type of diabetes medicine known as a sulfonylurea
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of CYCLOSET. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store CYCLOSET?
Store and dispense: At 68-77°F (20-25°C) in a tight, light-resistant container. See USP Controlled Room Temperature.
Keep CYCLOSET and all medicines out of the reach of children.
General information about the use of CYCLOSET
Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets. Do not use CYCLOSET for a condition for which it was not prescribed. Do not give CYCLOSET to other people, even if they have the same symptoms you have. It may harm them.
This leaflet summarizes the most important information about CYCLOSET. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for additional information about CYCLOSET that is written for health professionals. For more information, go to www.CYCLOSET.com or call 1-800-321-4576.
What are the ingredients in CYCLOSET?
Active ingredient: bromocriptine mesylate
Inactive ingredients: lactose, corn starch, magnesium stearate, colloidal silicon dioxide, and citric acid.
Manufactured for:
VeroScience, LLC
Tiverton, RI 02878 USADistributed by:
Salix Pharmaceuticals, a division of
Bausch Health US, LLC
Bridgewater, NJ 08807 USAFor information for healthcare professionals, call 1-800-321-4576.
For patent information:
http://veroscience.com/products/patents.htmlCycloset is a registered trademark of VeroScience LLC, Tiverton, RI 02878 USA used under license.
Revised: 02/2019
9625401 70014086 - PRINCIPAL DISPLAY PANEL - 0.8 mg Tablet Bottle Label
-
INGREDIENTS AND APPEARANCE
CYCLOSET
bromocriptine mesylate tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 73515-123 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength bromocriptine mesylate (UNII: FFP983J3OD) (bromocriptine - UNII:3A64E3G5ZO) bromocriptine 0.8 mg Inactive Ingredients Ingredient Name Strength lactose, unspecified form (UNII: J2B2A4N98G) starch, corn (UNII: O8232NY3SJ) magnesium stearate (UNII: 70097M6I30) silicon dioxide (UNII: ETJ7Z6XBU4) citric acid monohydrate (UNII: 2968PHW8QP) Product Characteristics Color white (white) Score no score Shape ROUND (ROUND) Size 6mm Flavor Imprint Code C;9 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 73515-123-21 21 in 1 BOTTLE; Type 0: Not a Combination Product 11/15/2010 2 NDC: 73515-123-30 200 in 1 BOTTLE; Type 0: Not a Combination Product 11/15/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020866 11/15/2010 Labeler - VEROSCIENCE LLC (556283799) Establishment Name Address ID/FEI Business Operations Patheon Pharmaceuticals Inc. 005286822 MANUFACTURE(73515-123) Establishment Name Address ID/FEI Business Operations AMRI Italy S.r.l. 440365767 API MANUFACTURE(73515-123)
Trademark Results [CYCLOSET]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CYCLOSET 86678707 not registered Dead/Abandoned |
VeroScience LLC 2015-06-30 |
 CYCLOSET 85482916 not registered Dead/Abandoned |
VeroScience LLC 2011-11-29 |
 CYCLOSET 85078890 3999613 Live/Registered |
VeroScience LLC 2010-07-06 |
 CYCLOSET 78976460 3016368 Live/Registered |
VEROSCIENCE LLC 2002-10-23 |
 CYCLOSET 78962930 not registered Dead/Abandoned |
VeroScience LLC 2006-08-29 |
 CYCLOSET 78177390 not registered Dead/Abandoned |
PLIVA, INC. 2002-10-23 |
 CYCLOSET 75572929 not registered Dead/Abandoned |
JOHNSON & JOHNSON 1998-10-19 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.