e.l.f Whoa Glow Broad Spectrum SPF30 Sunscreen
e.l.f Whoa Glow Broad Spectrum SPF30 Sunscreen by
Drug Labeling and Warnings
e.l.f Whoa Glow Broad Spectrum SPF30 Sunscreen by is a Otc medication manufactured, distributed, or labeled by e.l.f. Cosmetics, Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
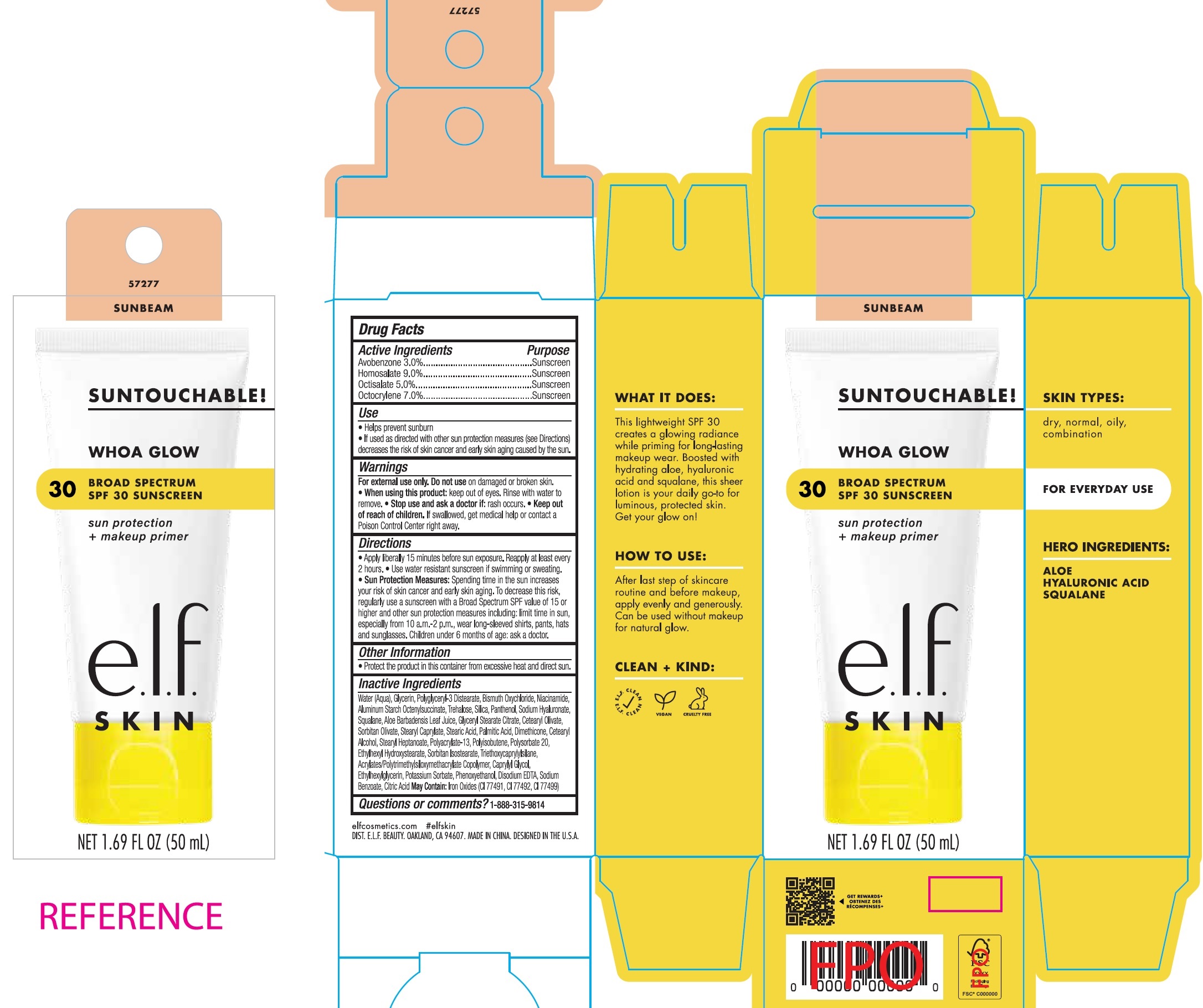
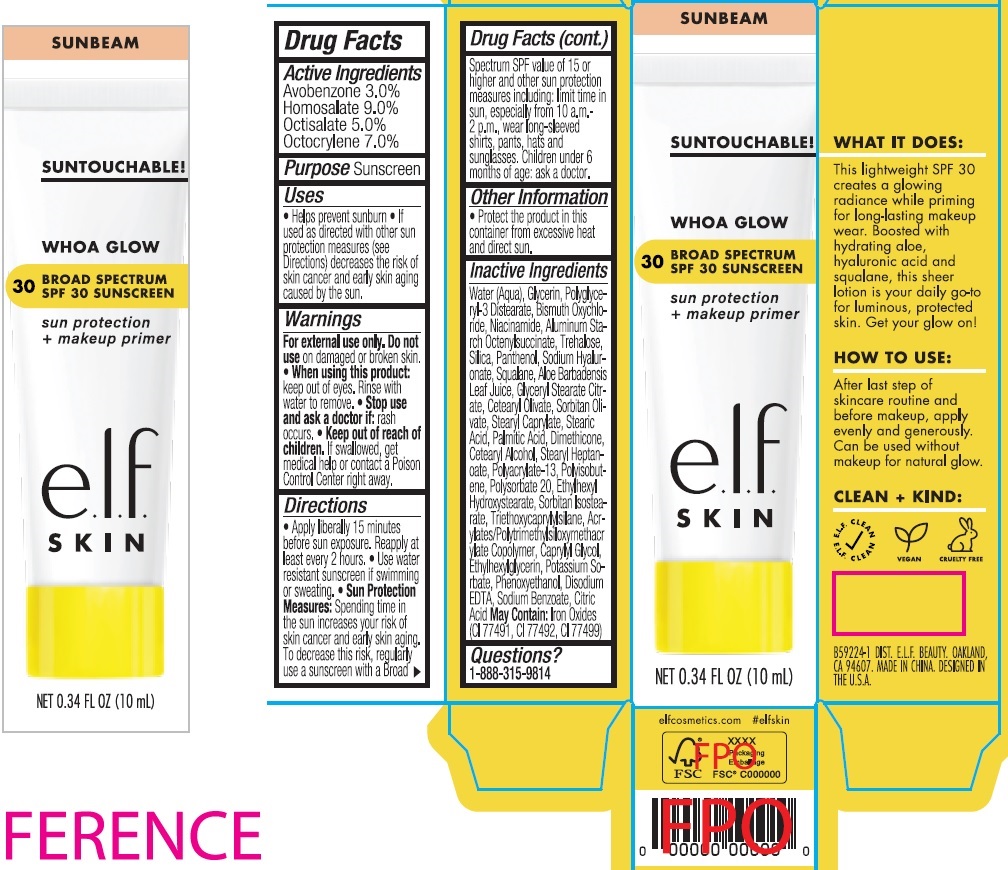
E.L.F WHOA GLOW BROAD SPECTRUM SPF30 SUNSCREEN- avobenzone, homosalate, octisalate, octocrylene cream
e.l.f. Cosmetics, Inc
----------
e.l.f Whoa Glow Broad Spectrum SPF30 Sunscreen
Use
- Helps prevent sunburn
- If used as directed with other sun protection measures (see Directions) decreases the risk of skin cancer and early skin aging caused by the sun.
Directions
- Apply liberally 15 minutes before sun exposure. Reapply at least every 2 hours.
- Use water resistant sunscreen if swimming or sweating.
- Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including: limit time in sun, especially from 10 a.m.-2 p.m., wear long-sleeved shirts, pants, hats and sunglasses. Children under 6 months of age: ask a doctor. Sun Protection Measures:
Inactive Ingredients
Water (Aqua), Glycerin, Polyglyceryl-3 Distearate, Bismuth Oxychloride, Niacinamide, Aluminum Starch Octenylsuccinate, Trehalose, Silica, Panthenol, Sodium Hyaluronate, Squalane, Aloe Barbadensis Leaf Juice, Glyceryl Stearate Citrate, Cetearyl Olivate, Sorbitan Olivate, Stearyl Caprylate, Stearic Acid, Palmitic Acid, Dimethicone, Cetearyl Alcohol, Stearyl Heptanoate, Polyacrylate-13, Polyisobutene, Polysorbate 20, Ethylhexyl Hydroxystearate, Sorbitan Isostearate, Triethoxycaprylylsilane, Acrylates/Polytrimethylsiloxymethacrylate Copolymer, Caprylyl Glycol, Ethylhexylglycerin, Potassium Sorbate, Phenoxyethanol, Disodium EDTA, Sodium Benzoate, Citric Acid May Contain: Iron Oxides (CI 77491, CI 77492, CI 77499)
| E.L.F WHOA GLOW BROAD SPECTRUM SPF30 SUNSCREEN
avobenzone, homosalate, octisalate, octocrylene cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - e.l.f. Cosmetics, Inc (093902816) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.