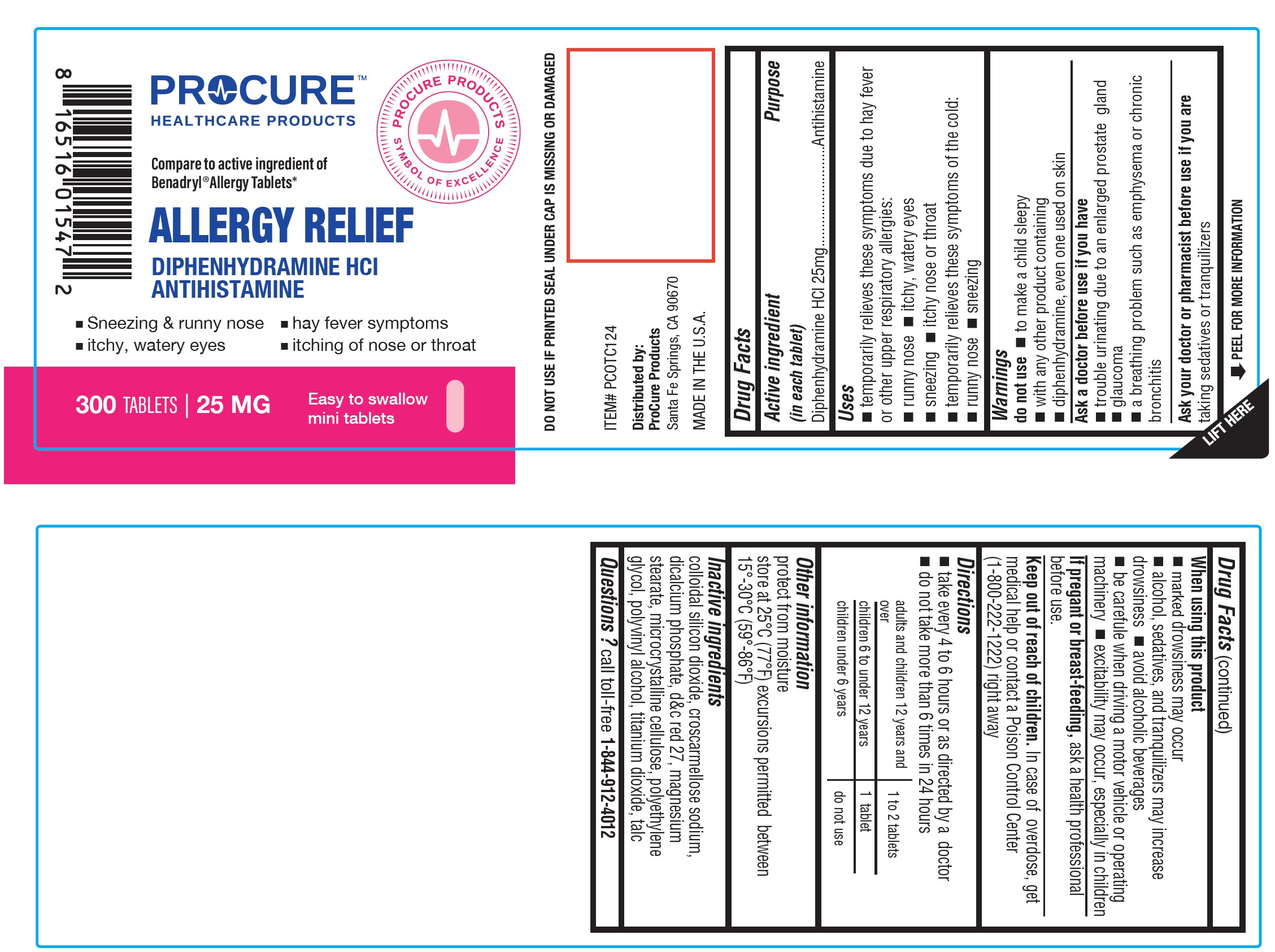
Twin Med - DIPHENHYDRAMINE HCl Tablets, USP 25mg (55681-322)
Allergy Relief by
Drug Labeling and Warnings
Allergy Relief by is a Otc medication manufactured, distributed, or labeled by TWIN MED LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ALLERGY RELIEF- diphenhydramine hydrochloride tablet
TWIN MED LLC
----------
Twin Med - DIPHENHYDRAMINE HCl Tablets, USP 25mg (55681-322)
Uses
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itchy nose or throat
- temporarily relieves these symptoms of the common cold:
- runny nose
- sneezing
Warnings
Do not use
- with any other product containing diphenhydramine, even one used on skin
- to make a child sleepy
Ask a doctor before use if you have
- trouble urinating due to an enlarged prostate gland
- glaucoma ? a breathing problem such as emphysema or chronic bronchitis
Ask a doctor or pharmacist before use if youaretaking sedatives or tranquilizers
When using this product
- marked drowsiness may occur
- avoid alcoholic drinks
- excitability may occur, especially in children ? alcohol, sedatives and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
Keep out of reach of children.
In case of accidental overdose, contact a doctor or Poison Control Center immediately. Prompt medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Do not exceed recommended dosage.
Directions
- take every 4 to 6 hours, not more than 6 doses in 24 hours
- Adults and children 12 years of age and older:1 or 2 tablets
- children 6 to under 12 years of age:1 tablet
- children 4 to under 6 years of age:do not use unless directed by a doctor
- children under 4 years of age:do not use
Other Information
- each tablet contains : calcium 20 mg
- store at controlled room temperature 20°-25°C (68°-77°F).
- read all product information before using.
- TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING.
| ALLERGY RELIEF
diphenhydramine hydrochloride tablet |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - TWIN MED LLC (009579330) |
Revised: 1/2026
<
Document Id: 47e2c140-edde-4a38-e063-6294a90a16b8
Set id: e4a47131-8017-3d92-e053-2a95a90a619e
Version: 4
Effective Time: 20260108
Trademark Results [Allergy Relief]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ALLERGY RELIEF 98236984 not registered Live/Pending |
Dmytro Kononenko 2023-10-24 |
 ALLERGY RELIEF 90457167 not registered Live/Pending |
American Textile Company, Inc. 2021-01-10 |
 ALLERGY RELIEF 78838437 3358249 Live/Registered |
Meshbesher Health Corporation 2006-03-16 |
 ALLERGY RELIEF 76619855 3066888 Live/Registered |
AMERICAN TEXTILE COMPANY 2004-11-09 |
 ALLERGY RELIEF 74668018 not registered Dead/Abandoned |
NaturaLife Corporation 1995-05-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.