POTELIGEO- mogamulizumab-kpkc injection
POTELIGEO by
Drug Labeling and Warnings
POTELIGEO by is a Prescription medication manufactured, distributed, or labeled by Kyowa Kirin, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use POTELIGEO safely and effectively. See full prescribing information for POTELIGEO.
POTELIGEO® (mogamulizumab-kpkc) injection, for intravenous use
Initial U.S. Approval: 2018INDICATIONS AND USAGE
POTELIGEO is a CC chemokine receptor type 4 (CCR4)-directed monoclonal antibody indicated for the treatment of adult patients with relapsed or refractory mycosis fungoides or Sézary syndrome after at least one prior systemic therapy [1].
DOSAGE AND ADMINISTRATION
1 mg/kg as an intravenous infusion over at least 60 minutes on days 1, 8, 15, and 22 of the first 28-day cycle and on days 1 and 15 of each subsequent cycle [2].
DOSAGE FORMS AND STRENGTHS
Injection: 20 mg/5 mL (4 mg/mL) solution in a single-dose vial [3].
CONTRAINDICATIONS
None [4].
WARNINGS AND PRECAUTIONS
- Dermatologic Toxicity: Temporarily interrupt POTELIGEO for moderate or severe skin rashes. Permanently discontinue POTELIGEO for life-threatening rash [5.1].
- Infusion Reactions: Temporarily interrupt POTELIGEO for any infusion reaction. Permanently discontinue POTELIGEO for any life-threatening infusion reaction [5.2].
- Infections: Monitor and treat promptly [5.3].
- Autoimmune Complications: Interrupt or permanently discontinue POTELIGEO as appropriate [5.4].
- Complications of Allogeneic HSCT after POTELIGEO: Monitor for severe acute graft-versus-host disease (GVHD) and steroid-refractory GVHD. Transplant-related mortality has occurred. [5.5].
ADVERSE REACTIONS
The most common adverse reactions (reported in ≥20% of patients) were rash, infusion related reactions, fatigue, diarrhea, musculoskeletal pain, and upper respiratory tract infection [6.1].
To report SUSPECTED ADVERSE REACTIONS, contact Kyowa Kirin, Inc. at 1-844-768-3544 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 8/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Dose Modifications for Toxicity
2.3 Preparation and Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Dermatologic Toxicity
5.2 Infusion Reactions
5.3 Infections
5.4 Autoimmune Complications
5.5 Complications of Allogeneic Hematopoietic Stem Cell Transplantation (HSCT) after POTELIGEO
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
6.2 Immunogenicity
6.3 Postmarketing Safety Information
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric use
8.5 Geriatric use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended dose of POTELIGEO is 1 mg/kg administered as an intravenous infusion over at least 60 minutes. Administer on days 1, 8, 15, and 22 of the first 28-day cycle, then on days 1 and 15 of each subsequent 28-day cycle until disease progression or unacceptable toxicity.
Administer POTELIGEO within 2 days of the scheduled dose. If a dose is missed, administer the next dose as soon as possible and resume dosing schedule.
Do not administer POTELIGEO subcutaneously or by rapid intravenous administration.
2.2 Dose Modifications for Toxicity
Dermatologic Toxicity
- Permanently discontinue POTELIGEO for life-threatening (Grade 4) rash or for any Stevens-Johnson syndrome (SJS) or toxic epidermal necrolysis (TEN) [see Warnings and Precautions (5.1)]. If SJS or TEN is suspected, stop POTELIGEO and do not resume unless SJS or TEN has been excluded and the cutaneous reaction has resolved to Grade 1 or less.
- If moderate or severe (Grades 2 or 3) rash occurs, interrupt POTELIGEO and administer at least 2 weeks of topical corticosteroids. If rash improves to Grade 1 or less, POTELIGEO may be resumed [see Warnings and Precautions (5.1)].
- If mild (Grade 1) rash occurs, consider topical corticosteroids.
Infusion Reactions
- Permanently discontinue POTELIGEO for a life-threatening (Grade 4) infusion reaction [see Warnings and Precautions (5.2)].
- Temporarily interrupt the infusion of POTELIGEO for mild to severe (Grades 1 to 3) infusion reactions and treat symptoms. Reduce the infusion rate by at least 50% when restarting the infusion after symptoms resolve. If reaction recurs and is unmanageable, discontinue infusion. [see Warnings and Precautions (5.2)].
- If an infusion reaction occurs, administer premedication (such as diphenhydramine and acetaminophen) for subsequent POTELIGEO infusions.
2.3 Preparation and Administration
Preparation
- Visually inspect drug product solution for particulate matter and discoloration prior to administration. POTELIGEO is a clear to slightly opalescent colorless solution. Discard the vial if cloudiness, discoloration, or particulates are observed.
- Calculate the dose (mg/kg) and number of vials of POTELIGEO needed to prepare the infusion solution based on patient weight.
- Aseptically withdraw the required volume of POTELIGEO into the syringe and transfer into an intravenous (IV) bag containing 0.9% Sodium Chloride Injection, USP. The final concentration of the diluted solution should be between 0.1 mg/mL to 3.0 mg/mL.
- Mix diluted solution by gentle inversion. Do not shake.
- Discard any unused portion left in the vial.
The diluted solution is compatible with polyvinyl chloride (PVC) or polyolefin (PO) infusion bags.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Dermatologic Toxicity
Fatal and life-threatening skin adverse reactions, including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), have occurred in recipients of POTELIGEO. Rash (drug eruption) is one of the most common adverse reactions associated with POTELIGEO. In Trial 1, 25% (80/319) of patients treated with POTELIGEO had an adverse reaction of drug eruption, with 18% of these cases being severe (Grade 3) and 82% of these cases being Grade 1 or 2. Of 528 patients treated with POTELIGEO in clinical trials, Grade 3 skin adverse reactions were reported in 3.6%, Grade 4 skin adverse reactions in <1%, and SJS in <1%.
The onset of drug eruption is variable, and the affected areas and appearance vary. In Trial 1, the median time to onset was 15 weeks, with 25% of cases occurring after 31 weeks. The more common presentations reported included papular or maculopapular rash, lichenoid, spongiotic or granulomatous dermatitis, and morbilliform rash. Other presentations included scaly plaques, pustular eruption, folliculitis, non-specific dermatitis, and psoriasiform dermatitis.
Monitor patients for rash throughout the treatment course. Management of dermatologic toxicity includes topical corticosteroids and interruption or permanent cessation of POTELIGEO [see Dosage and Administration (2.2)]. Consider skin biopsy to help distinguish drug eruption from disease progression.
Discontinue POTELIGEO permanently for SJS or TEN or for any life-threatening (Grade 4) reaction. For possible SJS or TEN, interrupt POTELIGEO and do not restart unless SJS or TEN is ruled out and the cutaneous reaction has resolved to Grade 1 or less.
5.2 Infusion Reactions
Fatal and life-threatening infusion reactions have been reported in patients treated with POTELIGEO. In Trial 1, infusion reactions occurred in 35% (112/319) of patients treated with POTELIGEO, with 8% of these reactions being severe (Grade 3). Most reactions (approximately 90%) occur during or shortly after the first infusion. Infusion reactions can also occur with subsequent infusions. The most commonly reported signs include chills, nausea, fever, tachycardia, rigors, headache, and vomiting.
Consider premedication (such as diphenhydramine and acetaminophen) for the first infusion of POTELIGEO in all patients. Whether premedication reduces the risk or severity of these reactions is not established. In Trial 1, infusion reactions occurred in 42% of patients without premedication and 32% of patients with premedication. Monitor patients closely for signs and symptoms of infusion reactions and interrupt the infusion for any grade reaction and treat promptly [see Dosage and Administration (2.2)].
5.3 Infections
Fatal and life-threatening infections have occurred in patients treated with POTELIGEO, including sepsis, pneumonia, and skin infection. In Trial 1, 18% (34/184) of patients randomized to POTELIGEO had Grade 3 or higher infection or an infection-related serious adverse reaction. Monitor patients for signs and symptoms of infection and treat promptly.
5.4 Autoimmune Complications
Fatal and life-threatening immune-mediated complications have been reported in recipients of POTELIGEO. Grade 3 or higher immune-mediated or possibly immune-mediated reactions have included myositis, myocarditis, polymyositis, hepatitis, pneumonitis, and a variant of Guillain-Barré syndrome. Use of systemic immunosuppressants for immune-mediated reactions was reported in 1.9% (6/319) of recipients of POTELIGEO in Trial 1, including for a case of Grade 2 polymyalgia rheumatica. New-onset hypothyroidism (Grade 1 or 2) was reported in 1.3% of patients and managed with observation or levothyroxine. Interrupt or permanently discontinue POTELIGEO as appropriate for suspected immune-mediated adverse reactions. Consider the benefit/risk of POTELIGEO in patients with a history of autoimmune disease.
5.5 Complications of Allogeneic Hematopoietic Stem Cell Transplantation (HSCT) after POTELIGEO
Increased risks of transplant complications have been reported in patients who receive allogeneic HSCT after POTELIGEO including severe (Grade 3 or 4) acute graft-versus-host disease (GVHD), steroid-refractory GVHD, and transplant-related death. Among recipients of pre-transplantation POTELIGEO, a higher risk of transplant complications has been reported if POTELIGEO is given within a shorter time frame (approximately 50 days) before HSCT. Follow patients closely for early evidence of transplant-related complications.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed in greater detail in other sections of the labeling:
- Dermatologic Toxicity [see Warnings and Precautions (5.1)].
- Infusion Reactions [see Warnings and Precautions (5.2)].
- Infections [see Warnings and Precautions (5.3)].
- Autoimmune Complications [see Warnings and Precautions (5.4)].
- Complications of Allogeneic HSCT after POTELIGEO [see Warnings and Precautions (5.5)].
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Trial 1
The data described below reflect exposure to POTELIGEO in a randomized, open-label, actively controlled clinical trial for adult patients with MF or SS who received at least one prior systemic therapy [see Clinical Studies (14)]. Of 370 patients treated, 184 (57% with MF, 43% with SS) received POTELIGEO as randomized treatment and 186 (53% with MF, 47% with SS) received vorinostat. In the vorinostat arm, 135 patients (73%) subsequently crossed over to POTELIGEO for a total of 319 patients treated with POTELIGEO.
POTELIGEO was administered at 1 mg/kg intravenously over at least 60 minutes on days 1, 8, 15, and 22 of the first 28-day cycle and on days 1 and 15 of subsequent 28-day cycles. Premedication (diphenhydramine, acetaminophen) was optional and administered to 65% of randomized patients for the first infusion. The comparator group received vorinostat 400 mg orally once daily, given continuously in 28-day cycles. Treatment continued until unacceptable toxicity or progressive disease.
The median age was 64 years (range, 25 to 101 years), 58% of patients were male, 70% were white, and 99% had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Patients had a median of 3 prior systemic therapies. The trial required an absolute neutrophil count (ANC) ≥1500/µL (≥1000/µL if bone marrow was involved), platelet count ≥100,000/µL (≥75,000/µL if bone marrow was involved), creatinine clearance >50 mL/min or serum creatinine ≤1.5 mg/dL, and hepatic transaminases ≤2.5 times upper limit of normal (ULN) (≤5 times ULN if lymphomatous liver infiltration). Patients with active autoimmune disease, active infection, autologous HSCT within 90 days, or prior allogeneic HSCT were excluded.
During randomized treatment, the median duration of exposure to POTELIGEO was 5.6 months, with 48% (89/184) of patients with at least 6 months of exposure and 23% (43/184) with at least 12 months of exposure. The median duration of exposure to vorinostat was 2.8 months, with 22% (41/186) of patients with at least 6 months of exposure.
Fatal adverse reactions within 90 days of the last dose occurred in 2.2% (7/319) of patients who received POTELIGEO as randomized or crossover treatment.
Serious adverse reactions were reported in 36% (66/184) of patients randomized to POTELIGEO and most often involved infection (16% of patients; 30/184). Serious adverse reactions reported in >2% of patients randomized to POTELIGEO were pneumonia (5%), sepsis (4%), pyrexia (4%), and skin infection (3%); other serious adverse reactions, each reported in 2% of patients, included hepatitis, pneumonitis, rash, infusion related reaction, lower respiratory tract infection, and renal insufficiency. POTELIGEO was discontinued for adverse reactions in 18% of randomized patients, most often due to rash or drug eruption (7.1%).
Common Adverse Reactions
The most common adverse reactions (reported in ≥20% of patients randomized to POTELIGEO) were rash (including drug eruption), infusion related reactions, fatigue, diarrhea, upper respiratory tract infection and musculoskeletal pain. Other common adverse reactions (reported in ≥10% of patients randomized to POTELIGEO) included skin infection, pyrexia, nausea, edema, thrombocytopenia, headache, constipation, mucositis, anemia, cough and hypertension. Table 1 summarizes common adverse reactions having a ≥2% higher incidence with POTELIGEO than with vorinostat in Trial 1.
Table 1: Common Adverse Reactions (≥10%) with ≥2% Higher Incidence in the POTELIGEO Arm Adverse Reactions by Body System *, † POTELIGEO
(N=184)Vorinostat
(N=186)All Grades
(%)≥Grade 3
(%)All Grades
(%)≥Grade 3
(%)Rash/Drug Eruption includes: dermatitis (allergic, atopic, bullous, contact, exfoliative, infected), drug eruption, palmoplantar keratoderma, rash (generalized, macular, maculopapular, papular, pruritic, pustular), skin reaction, toxic skin eruption
Upper Respiratory Tract Infection includes: laryngitis viral, nasopharyngitis, pharyngitis, rhinitis, sinusitis, upper respiratory tract infection, viral upper respiratory tract infection
Skin Infection includes: cellulitis, dermatitis infected, erysipelas, impetigo, infected skin ulcer, periorbital cellulitis, skin bacterial infection, skin infection, staphylococcal skin infection
Musculoskeletal Pain includes: back pain, bone pain, musculoskeletal chest pain, musculoskeletal pain, myalgia, neck pain, pain in extremity
Mucositis includes: aphthous stomatitis, mouth ulceration, mucosal inflammation, oral discomfort, oral pain, oropharyngeal pain, stomatitis- * Adverse reactions include groupings of individual preferred terms.
- † Includes adverse reactions reported up to 90 days after randomized treatment.
Skin and Subcutaneous Tissue Disorders Rash, Including Drug Eruption 35 5 11 2 Drug Eruption 24 5 <1 0 Procedural Complications Infusion Related Reaction 33 2 0 0 Infections Upper Respiratory Tract Infection 22 0 16 1 Skin Infection 19 3 13 4 Musculoskeletal and Connective Tissue Disorders Musculoskeletal Pain 22 <1 17 3 General Disorders Pyrexia 17 <1 7 0 Gastrointestinal Mucositis 12 1 6 0 Other Common Adverse Reactions in ≥10% of POTELIGEO Arm 1, 2
- General disorders: fatigue (31%), edema (16%)
- Gastrointestinal disorders: diarrhea (28%), nausea (16%), constipation (13%)
- Blood and lymphatic system disorders: thrombocytopenia (14%), anemia (12%)
- Nervous system disorders: headache (14%)
- Vascular disorders: hypertension (10%)
- Respiratory disorders: cough (11%)
Adverse Reactions in ≥5% but <10% of POTELIGEO Arm 1, 2
- Infections: candidiasis (9%), urinary tract infection (9%), folliculitis (8%), pneumonia (6%), otitis (5%), herpesvirus infection (5%)
- Investigations: renal insufficiency (9%), hyperglycemia (9%), hyperuricemia (8%), weight increase (8%), weight decrease (6%), hypomagnesemia (6%)
- Psychiatric disorders: insomnia (9%), depression (7%)
- Skin and subcutaneous disorders: xerosis (8%), alopecia (7%)
- Nervous system disorders: dizziness (8%), peripheral neuropathy (7%)
- Metabolism and nutrition disorders: decreased appetite (8%)
- Respiratory disorders: dyspnea (7%)
- General disorders: chills (7%)
- Gastrointestinal disorders: vomiting (7%), abdominal pain (5%)
- Injury, poisoning and procedural complications: fall (6%)
- Musculoskeletal disorders: muscle spasms (5%)
- Cardiovascular disorders: arrhythmia (5%)
- Eye disorders: conjunctivitis (5%)
Selected Other Adverse Reactions 1, 2
- Tumor lysis syndrome (<1%)
- Myocardial ischemia or infarction (<1%)
- Cardiac failure (<1%)
Table 2 summarizes common treatment-emergent laboratory abnormalities having a ≥2% higher incidence with POTELIGEO than with vorinostat.
Table 2: Common New or Worsening Laboratory Abnormalities (≥10%) with ≥2% Higher Incidence in the POTELIGEO Arm Laboratory Test * POTELIGEO
(N=184)Vorinostat
(N=186)All Grades
(%)≥Grade 3
(%)All Grades
(%)≥Grade 3
(%)- * Includes laboratory abnormalities, reported up to 90 days after treatment, that are new or worsening in grade or with worsening from baseline unknown.
- † Out of 99 evaluable recipients of POTELIGEO and 36 evaluable recipients of vorinostat.
Chemistry Albumin Decreased 34 2 27 3 Calcium Decreased 30 3 20 2 Uric Acid Increased 29 29 11 11 Phosphate Decreased 27 5 26 5 Magnesium Decreased 17 <1 8 <1 Glucose Decreased 14 0 8 <1 Calcium Increased 12 <1 8 <1 Hematology CD4 Lymphocytes Decreased † 63 43 17 8 Lymphocytes Decreased 31 16 12 4 White Blood Cells Decreased 33 2 18 2 Other common treatment-emergent laboratory abnormalities in the POTELIGEO arm included hyperglycemia (52%; 4% Grade 3-4), anemia (35%; 2% Grade 3-4), thrombocytopenia (29%, none Grade 3-4), aspartate transaminase (AST) increased (25%; 2% Grade 3-4), alanine transaminase (ALT) increased (18%; 1% Grade 3-4), alkaline phosphatase increased (17%; 0% Grade 3-4), and neutropenia (10%; 2% Grade 3-4). Grade 4 treatment-emergent laboratory abnormalities observed in ≥1% of the POTELIGEO arm included lymphopenia (5%), leukopenia (1%), and hypophosphatemia (1%).
- 1 Includes grouped terms
- 2 From 184 patients randomized to POTELIGEO
6.2 Immunogenicity
As with all therapeutic proteins, there is a potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors, including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of incidence of antibodies to POTELIGEO with the incidences of antibodies in other studies or to other products may be misleading.
Among 258 patients treated with POTELIGEO in Trial 1, 10 (3.9%) tested positive for treatment-emergent (treatment-induced or treatment-boosted) anti-mogamulizumab-kpkc antibodies by an electrochemiluminescent assay. There were no positive neutralizing antibody responses.
6.3 Postmarketing Safety Information
The following adverse reactions have been identified during post-approval use of POTELIGEO. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Infections: Hepatitis B virus reactivation
- Cardiac disorders: Stress cardiomyopathy
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on POTELIGEO use in pregnant women to inform a drug-associated risk of major birth defects and miscarriage. In an animal reproduction study, administration of mogamulizumab-kpkc to pregnant cynomolgus monkeys from the start of organogenesis through delivery did not show a potential for adverse developmental outcomes at maternal systemic exposures 27 times the exposure in patients at the recommended dose, based on AUC (see Data). In general, IgG molecules are known to cross the placental barrier and in the monkey reproduction study mogamulizumab-kpkc was detected in fetal plasma. Therefore, POTELIGEO has the potential to be transmitted from the mother to the developing fetus. POTELIGEO is not recommended during pregnancy or in women of childbearing potential not using contraception.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risks of major birth defects and miscarriage in clinically recognized pregnancies are 2-4% and 15-20%, respectively.
Data
Animal Data
The effects of mogamulizumab-kpkc on embryo-fetal development were evaluated in 12 pregnant cynomolgus monkeys that received mogamulizumab-kpkc once weekly by intravenous administration from the start of organogenesis through delivery at an exposure level 27 times higher than the clinical dose. Mogamulizumab-kpkc administration did not show a potential for embryo-fetal lethality, teratogenicity, or fetal growth retardation and did not result in spontaneous abortion or increased fetal death. In surviving fetuses (10 of 12 compared with 11 of 12 in the control group) of cynomolgus monkeys treated with mogamulizumab-kpkc, a decrease in CCR4-expressing lymphocytes due to the pharmacological activity of mogamulizumab-kpkc was noted; there were no apparent mogamulizumab-kpkc -related external, visceral, or skeletal abnormalities.
8.2 Lactation
Risk Summary
There is no information regarding the presence of POTELIGEO in human milk, the effects on the breastfed child, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for POTELIGEO and any potential adverse effects on the breastfed child from POTELIGEO or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
POTELIGEO is not recommended during pregnancy or in women of childbearing potential not using contraception.
8.4 Pediatric use
The safety and effectiveness of POTELIGEO in pediatric patients have not been established.
8.5 Geriatric use
Of 319 patients with MF or SS who received POTELIGEO in Trial 1, 162 (51%) were ≥65 years. No overall differences in effectiveness were observed between these patients and younger patients. In patients aged ≥65, Grade 3 or higher adverse reactions were reported in 45% and serious adverse reactions in 36%, whereas in patients aged <65, Grade 3 or higher adverse reactions were reported in 36% and serious adverse reactions in 29%.
-
11 DESCRIPTION
Mogamulizumab-kpkc is a recombinant humanized monoclonal antibody that targets CC chemokine receptor 4 (CCR4)-expressing cells. Mogamulizumab-kpkc is an IgG1 kappa immunoglobulin that has a calculated molecular mass of approximately 149 kDa. Mogamulizumab-kpkc is produced by recombinant DNA technology in Chinese hamster ovary cells.
POTELIGEO (mogamulizumab-kpkc) injection is a sterile, ready-to-use, preservative-free, clear to slightly opalescent colorless solution in a single-dose vial for dilution prior to intravenous infusion. Each vial contains 20 mg of mogamulizumab-kpkc in 5 mL of solution. Each mL of solution contains 4 mg of mogamulizumab-kpkc and is formulated in: citric acid monohydrate (0.44 mg), glycine (22.5 mg), polysorbate 80 (0.2 mg), and Water for Injection, USP. May contain hydrochloric acid/sodium hydroxide to adjust pH to 5.5.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Mogamulizumab-kpkc is a defucosylated, humanized IgG1 kappa monoclonal antibody that binds to CCR4, a G protein-coupled receptor for CC chemokines that is involved in the trafficking of lymphocytes to various organs. Non-clinical in vitro studies demonstrate mogamulizumab-kpkc binding targets a cell for antibody-dependent cellular cytotoxicity (ADCC) resulting in depletion of the target cells. CCR4 is expressed on the surface of some T-cell malignancies and is expressed on regulatory T-cells (Treg) and a subset of Th2 T-cells.
12.2 Pharmacodynamics
Mogamulizumab-kpkc exposure-response relationships and the time course of pharmacodynamics response are unknown.
12.3 Pharmacokinetics
Mogamulizumab-kpkc pharmacokinetics (PK) was evaluated in patients with T-cell malignancies. Parameters are presented as the geometric mean [% coefficient of variation (%CV)] unless otherwise specified. Mogamulizumab-kpkc concentrations increased proportionally with dose over the dose range of 0.01 to 1.0 mg/kg (0.01 to 1 times the approved recommended dosage).
Following repeated dosing of the approved recommended dosage, steady state concentrations were reached after 8 doses (12 weeks), and the systemic accumulation was 1.6-fold. At steady state, the peak concentration (Cmax,ss) is 32 (68%) µg/mL, the trough concentration (Cmin,ss) is 11 (239%) µg/mL, and AUCss is 5577 (125%) µg∙hr/mL.
Specific Populations:
No clinically significant changes in the PK of mogamulizumab-kpkc were observed based on age (range: 22 to 101 years), sex, ethnicity, renal impairment (creatinine clearance <90 mL/min, estimated by Cockcroft-Gault), mild (total bilirubin ≤ ULN and AST <ULN, or total bilirubin <1 to 1.5 times ULN and any AST) or moderate (total bilirubin >1.5 to 3 times ULN and any AST) hepatic impairment, disease subtype (MF or SS), degree of CCR4 expression, or ECOG status. The effect of severe hepatic impairment (total bilirubin >3 times ULN and any AST) on mogamulizumab-kpkc PK is unknown.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility
No carcinogenicity or genotoxicity studies have been conducted with POTELIGEO.
No specific studies have been conducted to evaluate potential effects of POTELIGEO on fertility. No mogamulizumab-kpkc -related toxic effects in the male and female reproductive organs were observed in sexually mature monkeys in repeat-dose toxicology studies up to 26 weeks in duration.
-
14 CLINICAL STUDIES
Trial 1
A randomized, open-label, multicenter trial (Study 0761-010; NCT01728805) evaluated the efficacy of POTELIGEO in adult patients with MF or SS after at least one prior systemic therapy. The trial randomized 372 patients 1:1 to either POTELIGEO (186 patients; 56% with MF, 44% with SS) or vorinostat (186 patients; 53% with MF, 47% with SS). The trial included patients regardless of tumor CCR4 expression status and excluded patients with histologic transformation, prior allogeneic HSCT, autologous HSCT within 90 days, active autoimmune disease, or active infection. The trial required patients to have ANC ≥1500/µL (≥1000/µL if bone marrow was involved), platelet count ≥100,000/µL (≥75,000/µL if bone marrow was involved), creatinine clearance >50 mL/min or serum creatinine ≤1.5 mg/dL and hepatic transaminases ≤2.5 times ULN (≤5 times ULN if lymphomatous liver infiltration).
The dose of POTELIGEO was 1 mg/kg administered intravenously over at least 60 minutes on days 1, 8, 15, and 22 of the first 28-day cycle and on days 1 and 15 of each subsequent cycle. Vorinostat was dosed at 400 mg orally once daily, continuously for 28-day cycles. Treatment continued until disease progression or unacceptable toxicity. Vorinostat-treated patients with disease progression or unacceptable toxicities were permitted to cross over to POTELIGEO.
The median age was 64 years (range: 25 to 101), 58% of patients were male, and 70% were white. At study baseline, 38% had stage IB-II disease, 10% stage III, and 52% stage IV. The median number of prior systemic therapies was 3. In the POTELIGEO arm, baseline CCR4 expression status by immunohistochemistry was available in 140 patients (75%), of whom all had CCR4 detected on ≥1% of lymphocytes on skin biopsy, and 134/140 (96%) had CCR4 detected on ≥10% of the lymphocytes. CCR4 expression status was similar in the vorinostat arm.
During randomized treatment, the median duration of exposure to POTELIGEO was 5.6 months (range: <1 to 45.3 months), with 48% of patients with at least 6 months of exposure and 23% with at least 12 months of exposure. The median duration of exposure to vorinostat was 2.8 months (range: <1 to 34.8 months), with 22% of patients with at least 6 months of exposure.
Efficacy was based on investigator-assessed progression-free survival (PFS), which was defined as the time from the date of randomization until documented progression of disease or death. Other efficacy measures included overall response rate (ORR) based on global composite response criteria that combine measures from each disease compartment (skin, blood, lymph nodes and viscera). Responses required confirmation at two successive disease assessments, which included the modified Severity Weighted Assessment Tool, skin photographs, central flow cytometry, and computed tomography.
The trial demonstrated that POTELIGEO significantly prolonged PFS compared to vorinostat (Table 3). The Kaplan-Meier curve for PFS by Investigator is shown in Figure 1. The estimated median follow-up for investigator-assessed PFS was 13 months in the POTELIGEO arm and 10.4 months in the vorinostat arm. By independent review committee assessment, the estimated median PFS was 6.7 months (95% CI, 5.6 to 9.4) in the POTELIGEO arm and 3.8 months (95% CI, 3.0 to 4.7) in the vorinostat arm (hazard ratio 0.64; 95% CI: 0.49, 0.84).
Figure 1 Kaplan-Meier Curve for Progression-Free Survival per Investigator
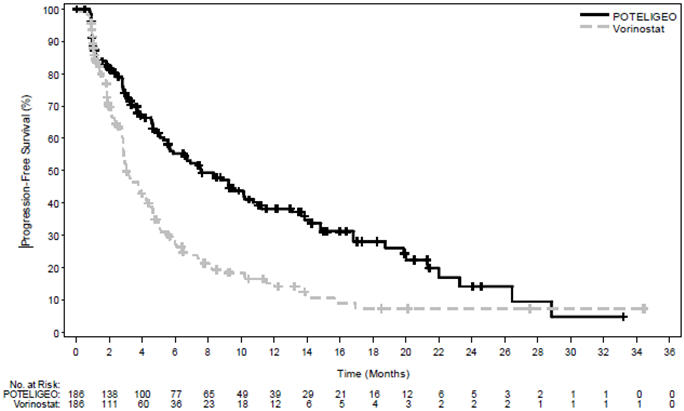
Table 3 also summarizes investigator-assessed confirmed response rates, overall and by disease compartment. The trial demonstrated improvement in ORR with POTELIGEO.
Table 3 Efficacy of Randomized Treatment (Trial 1) Outcome per Investigator POTELIGEO
N=186Vorinostat
N=186CI=confidence interval; CR=complete response; NE=not estimable; PR=partial response - * Kaplan-Meier estimate.
- † Based on Global Composite Response score.
- ‡ Responses in blood and skin must have persisted for at least 4 weeks to be considered confirmed and were evaluated every 4 weeks for the first year. Responses in lymph nodes, visceral disease and overall were evaluated every 8 weeks for the first year.
- § From Cochran-Mantel-Haenszel test adjusted for disease type, stage, and region.
PFS Number of events, n 110 131 Progressive disease 104 128 Death 6 3 Median PFS (95% CI) (months) * 7.6 (5.6, 10.2) 3.1 (2.8, 4.0) Hazard ratio (95% CI)
Log rank p-value0.53 (0.41, 0.69)
<.001Overall response rate (confirmed CR + PR), n (%) †, ‡ 52 (28) 9 (5) 95% CI (22, 35) (2, 9) P-value § <.001 Duration of overall response (months) Median (95% CI) * 13.9 (9.3, 18.9) 9.0 (4.6, NE) Confirmed best overall response † CR, n (%) 4 (2) 0 (0) 95% CI (1, 5) (0, 2) PR, n (%) 47 (25) 9 (5) 95% CI (20, 33) (2, 9) Response by compartment (confirmed CR + PR) ‡ Blood n=124 n=125 Response rate, n (%) 83 (67) 23 (18) 95% CI (58, 75) (12, 26) Skin n=186 n=186 Response rate, n (%) 78 (42) 29 (16) 95% CI (35, 49) (11, 22) Lymph nodes n=136 n=133 Response rate, n (%) 21 (15) 5 (4) 95% CI (10, 23) (1, 9) Viscera n=6 n=4 Response rate, n (%) 0 (0) 0 (0) 95% CI (0, 46) (0, 60) -
16 HOW SUPPLIED/STORAGE AND HANDLING
POTELIGEO (mogamulizumab-kpkc) injection is a sterile, preservative-free, clear to slightly opalescent colorless solution supplied in a carton containing one 20 mg/5 mL (4 mg/mL), single-dose glass vial (NDC: 42747-761-01).
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Inform patients of the risk of the following adverse reactions that may require additional treatment and/or withholding or discontinuation of POTELIGEO including:
- Dermatological Toxicity: Advise patients to contact their healthcare provider immediately for new or worsening skin rash [see Warnings and Precautions (5.1)]. Advise patients that the rash can happen at any time while receiving POTELIGEO.
- Infusion Reactions: Advise patients to contact their healthcare provider immediately for signs or symptoms of infusion reactions [see Warnings and Precautions (5.2)].
- Infections: Advise patients to contact their health care provider for fever or other evidence of infection [see Warnings and Precautions (5.3)].
- Autoimmune Complications: Advise patients to notify their healthcare provider of any history of autoimmune disease [see Warnings and Precautions (5.4)].
- Complications of Allogeneic HSCT after POTELIGEO: Advise patients of potential risk of post-transplant complications [see Warnings and Precautions (5.5)].
- Females of Reproductive Potential: Advise use of effective contraception during treatment with POTELIGEO and for at least 3 months following the last dose of POTELIGEO [see Use in Specific Populations (8.3)].
- SPL UNCLASSIFIED SECTION
-
PATIENT PACKAGE INSERT
-
PRINCIPAL DISPLAY PANEL - 4 mg/mL Vial Carton
Rx only
NDC: 42747-761-01
POTELIGEO®
(mogamulizumab-kpkc)Injection
20 mg/5 mL
(4 mg/mL)For Intravenous Infusion
Single-dose vial.
Discard unused portion.
-
INGREDIENTS AND APPEARANCE
POTELIGEO
mogamulizumab-kpkc injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 42747-761 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength mogamulizumab (UNII: YI437801BE) (mogamulizumab - UNII:YI437801BE) mogamulizumab 4 mg in 1 mL Inactive Ingredients Ingredient Name Strength citric acid monohydrate (UNII: 2968PHW8QP) 2.2 mg in 1 mL glycine (UNII: TE7660XO1C) 112.5 mg in 1 mL polysorbate 80 (UNII: 6OZP39ZG8H) 1 mg in 1 mL sodium hydroxide (UNII: 55X04QC32I) hydrochloric acid (UNII: QTT17582CB) water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42747-761-01 1 in 1 CARTON 08/08/2018 1 5 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761051 08/08/2018 Labeler - Kyowa Kirin, Inc. (014778321)
Trademark Results [POTELIGEO]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 POTELIGEO 79089360 4030555 Live/Registered |
Kyowa Kirin Co., Ltd. 2010-10-18 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.