Revesta by Sterling Knight Pharmaceuticals,LLC REVESTA capsule
Revesta by
Drug Labeling and Warnings
Revesta by is a Prescription medication manufactured, distributed, or labeled by Sterling Knight Pharmaceuticals,LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
Revesta is an orally administered prescription Vitamin for the dietary management of patients with unique nutritional needs requiring increased folate levels, Vitamin D supplementation due to Vitamin D deficiency and other nutritional supplementation.
Revesta should be administered under the supervision of a licensed medical practitioner.
Vitamin D3 (cholecalciferol) is a white, crystalline powder, very soluble in water, with the following structural formula:
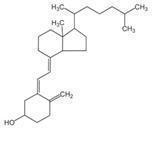
Each capsule contains:
Folic Acid: 1mg, Vitamin D3 (Cholecalciferol): 5750IUEach capsule contains the following inactive ingredients: soybean oil, gelatin (bovine), yellow wax, glycerin, deionized water, lecithin, titanium dioxide, FD&C Blue #1.
- INDICATIONS AND USAGE
-
CLINICAL PHARMACOLOGY
The in vivo synthesis of the major biologically active metabolites of vitamin D occurs in two steps. The first hydroxylation of ergocalciferol takes place in the liver (to 25-hydroxyvitamin D) and the second in the kidneys (to 1,25-dihydroxyvitamin D). Vitamin D metabolites promote the active absorption of calcium and phosphorus by the small intestine, thus elevating serum calcium and phosphate levels sufficiently to permit bone mineralization. Vitamin D metabolites also mobilize calcium and phosphate from bone and probably increase the reabsorption of calcium and perhaps also of phosphate by the renal tubules.
There is a time lag of 10 to 24 hours between the administration of vitamin D and the initiation of its action in the body due to the necessity of synthesis of the active metabolites in the liver and kidneys. Parathyroid hormone is responsible for the regulation of this metabolism in the kidneys.
- CONTRAINDICATIONS
-
WARNINGS AND PRECAUTIONS
Tell your doctor if you have: kidney problems, thyroid disease. This medication should be used as directed during pregnancy or while breast-feeding. Consult your doctor about the risks and benefits.
Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B12 is deficient. Folic acid in doses above 1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations progress.
-
ADVERSE REACTIONS
This medication is generally well tolerated. Notify your doctor if you experience: nausea, loss of appetite, vomiting, stomach cramps, dry mouth, increased thirst, increased urination, muscle or bone pain,headache, weakness, weight loss, dizziness. If you notice other effects not listed above, contact your doctor or pharmacist.
- DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING SECTION
-
OTHER SAFETY INFORMATION
Reserved for Professional Recommendation
All prescriptions using this product shall be pursuant to state statutes as applicable. This is not an Orange Book product. This product may be administered only under a physician’s supervision. There are no implied or explicit claims on therapeutic equivalence.
-
PACKAGE LABEL. PRINCIPAL DISPLAY PANEL
Rx Only
Reserved for Professional Recommendation
All prescriptions using this product shall be pursuant to state statutes as applicable. This is not an Orange Book product. This product may be administered only under a physician’s supervision. There are no implied or explicit claims on therapeutic equivalence.Manufactured for:Sterling Knight Pharmaceuticals, LLC
Ripley, MS 38663
Rev. 1114-1
-
INGREDIENTS AND APPEARANCE
REVESTA
revesta capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 69336-330 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 5750 [iU] FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 1 mg Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SOYBEAN OIL (UNII: 241ATL177A) YELLOW WAX (UNII: 2ZA36H0S2V) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) GLYCERIN (UNII: PDC6A3C0OX) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) GELATIN (UNII: 2G86QN327L) Product Characteristics Color blue Score no score Shape CAPSULE Size 22mm Flavor Imprint Code 330 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69336-330-30 30 in 1 BOTTLE, PLASTIC; Type 1: Convenience Kit of Co-Package 10/13/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 10/13/2016 Labeler - Sterling Knight Pharmaceuticals,LLC (079556942) Establishment Name Address ID/FEI Business Operations Sterling Knight Pharmaceuticals,LLC 079556942 manufacture(69336-330) , label(69336-330)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.