Golden Cup Balm 1950 by Golden Cup Pharmaceutical Co., Ltd. Golden Cup Balm 1950
Golden Cup Balm 1950 by
Drug Labeling and Warnings
Golden Cup Balm 1950 by is a Otc medication manufactured, distributed, or labeled by Golden Cup Pharmaceutical Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
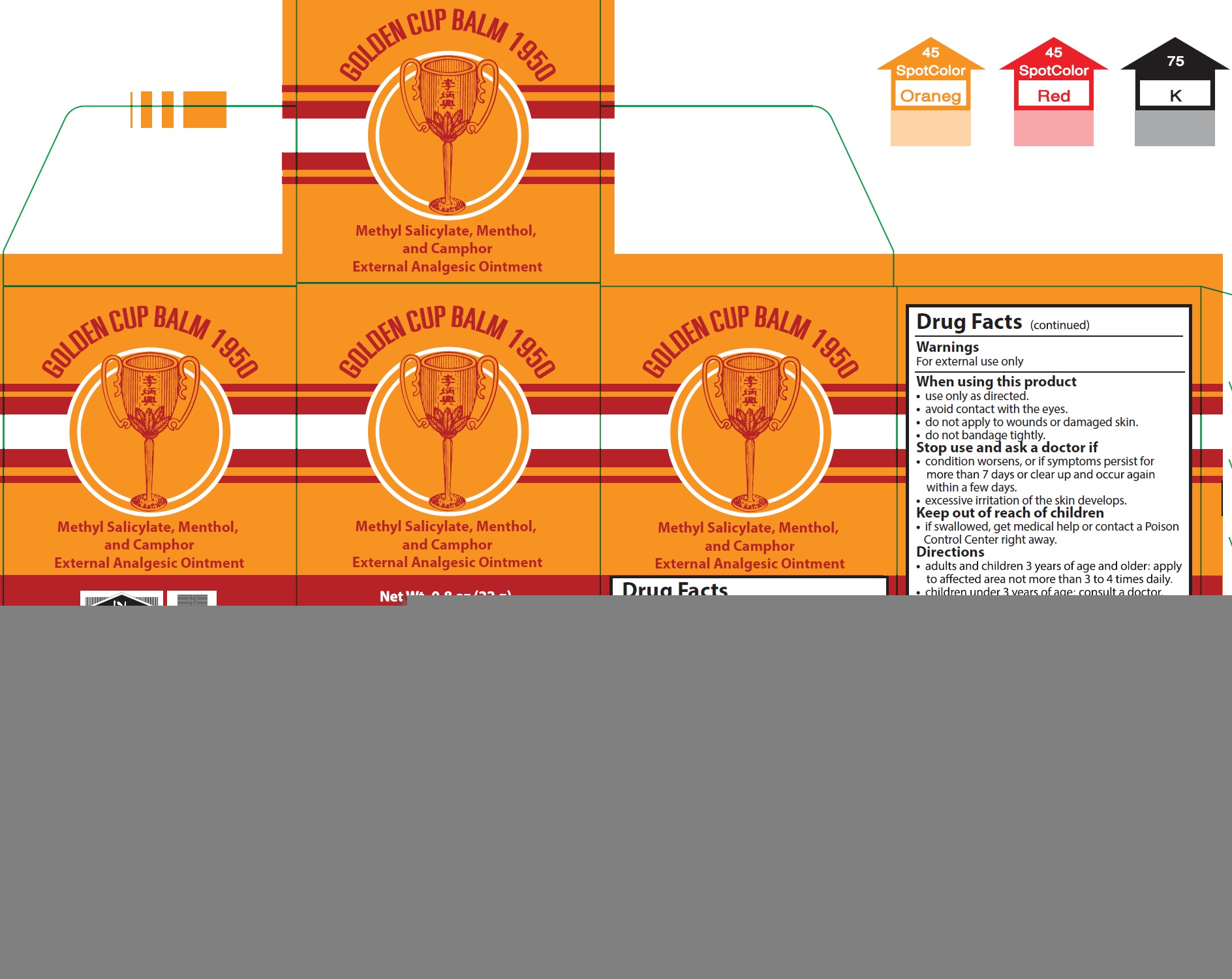
GOLDEN CUP BALM 1950- methyl salicylate, menthol, camphor (synthetic) ointment
Golden Cup Pharmaceutical Co., Ltd.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Golden Cup Balm 1950
Use
for the temporary relief of minor aches and pains of muscles and joints associated with simple backache, arthritis, strains, bruises, and sprains.
Warnings
For external use only
When using this product
- use only as directed.
- avoid contact with the eyes.
- do not apply to wounds or damaged skin,
- do not bandage tightly.
Directions
- adults and children 3 years of age and older: apply to affected area not more than 3 to 4 times daily.
- children under 3 years of age: consult a doctor.
| GOLDEN CUP BALM 1950
methyl salicylate, menthol, camphor (synthetic) ointment |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Golden Cup Pharmaceutical Co., Ltd. (659853253) |
Revised: 12/2022
Document Id: efdf596d-a5d2-2fb0-e053-2a95a90a613b
Set id: e561b934-094f-4ff6-9f6d-ccc4731c2845
Version: 2
Effective Time: 20221215