4TP - ORAL SOLUTION (82926-204) -DELIST
4TP ORAL by
Drug Labeling and Warnings
4TP ORAL by is a Homeopathic medication manufactured, distributed, or labeled by 4TP LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
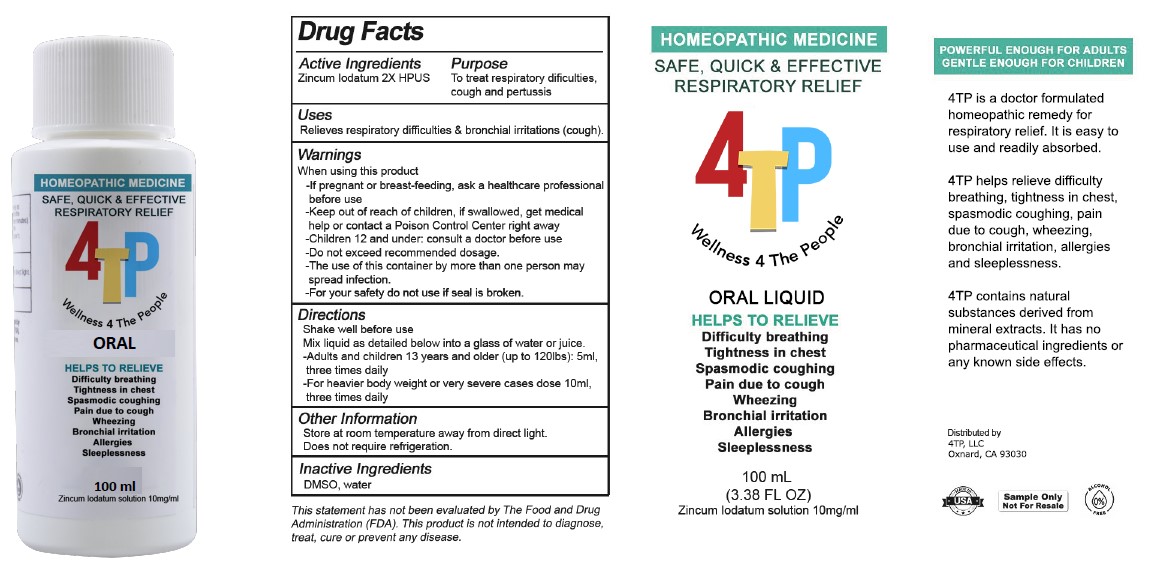
4TP ORAL- zincum iodatum solution
4TP LLC
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
4TP - ORAL SOLUTION (82926-204) -DELIST
WARNINGS
WHEN USING THIS PRODUCT
- If pregnant or breast-feeding, ask a healthcare professional before use
- Children under 12: consult a doctor before use
- Do not exceed recommended dosage
- The use of this container by more than one person may spread infection
- For your safety do not use if seal is broken
- Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control center right away
| 4TP ORAL
zincum iodatum solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - 4TP LLC (118753544) |
Revised: 3/2023
Document Id: f66db5c8-ceaa-38df-e053-2995a90a8bed
Set id: e56e8d41-188e-5a6f-e053-2a95a90a70e6
Version: 3
Effective Time: 20230308
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.