MARK. BLEMISH BANISHER ANTI-ACNE BODY TREATMENT PADS- salicylic acid cloth
MARK. BLEMISH BANISHER by
Drug Labeling and Warnings
MARK. BLEMISH BANISHER by is a Otc medication manufactured, distributed, or labeled by Avon Products, Inc. , Avon Products, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
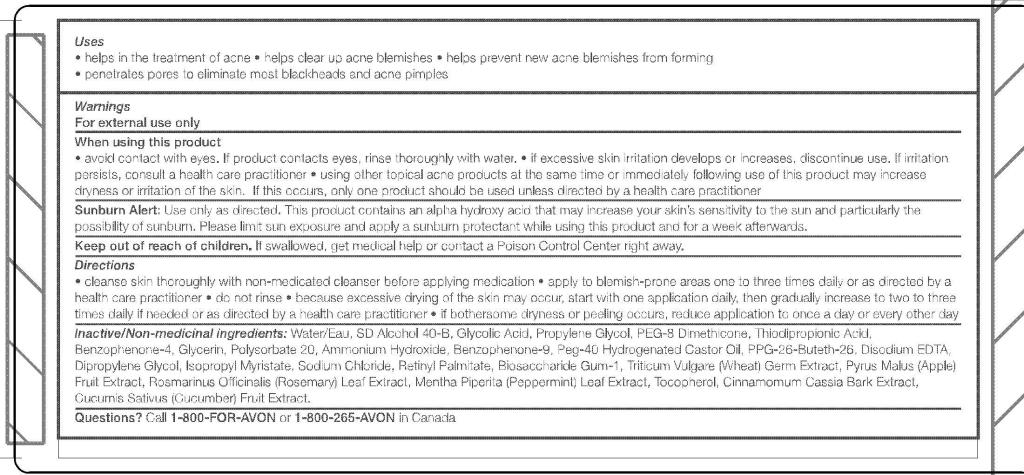
- ACTIVE INGREDIENT
- INDICATIONS & USAGE
-
WARNINGS
Warnings
For external use only
When using this product
- avoid contact with eyes. If product contacts eyes, rinse thoroughly with water.
- if excessive skin irritation develops or increases, discontinue use. If irritation persists, consult a health care practitioner
- using other topical acne products at the same time or immediately following use of this product may increase dryness or irritation of the skin. If this occurs, only one product should be used unless directed by a health care practitioner
Sunburn Alert: Use only as directed. This product contains alpha hydroxy acid that may increase you skin's sensitivity to the sun and particularly the possibility of sunburn. Please limit sun exposure and apply a sunburn protectant while using this product and for a week afterwards.
This product
-
DOSAGE & ADMINISTRATION
Directions
- cleanse skin thoroughly with non-medicated cleanser before applying medication
- apply to blemish-prone areas one to three times daily or as directed by a health care practitioner
- do not rinse
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two to three times daily if needed or as directed by a health care practitioner
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day
-
INACTIVE INGREDIENT
Inactive/Non-medicinal
ingredients
WATER/EAU
SD ALCOHOL 40-B
GLYCOLIC ACID
PROPYLENE GLYCOL
PEG-8 DIMETHICONE
THIODIPROPIONIC ACID
TRITICUM VULGARE (WHEAT) GERM EXTRACT
PYRUS MALUS (APPLE) FRUIT EXTRACT
ROSMARINUS OFFICINALIS (ROSEMARY) LEAF EXTRACT
MENTHA PIPERITA (PEPPERMINT) LEAF EXTRACT
CINNAMOMUM CASSIA BARK EXTRACT
CUCUMIS SATIVUS (CUCUMBER) FRUIT EXTRACT
TOCOPHEROL
BENZOPHENONE-4
GLYCERIN
POLYSORBATE 20
BENZOPHENONE-9
PEG-40 HYDROGENATED CASTOR OIL
PPG-26-BUTETH-26
DIPROPYLENE GLYCOL
ISOPROPYL MYRISTATE
SODIUM CHLORIDE
RETINYL PALMITATE
BIOSACCHARIDE GUM-1
AMMONIUM HYDROXIDE
DISODIUM EDTA
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MARK. BLEMISH BANISHER ANTI-ACNE BODY TREATMENT PADS
salicylic acid clothProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 10096-0194 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID .6 mL in 60 mL Inactive Ingredients Ingredient Name Strength GLYCOLIC ACID (UNII: 0WT12SX38S) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) GLYCERIN (UNII: PDC6A3C0OX) POLYSORBATE 20 (UNII: 7T1F30V5YH) DIPROPYLENE GLYCOL (UNII: E107L85C40) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 10096-0194-1 60 mL in 1 JAR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333 12/29/2009 Labeler - Avon Products, Inc. (001468693) Establishment Name Address ID/FEI Business Operations Avon Products, Inc. 001807106 manufacture
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.