Bath & Body Works, Inc. / Accel Inc. DRUG FACTS
Drug Labeling and Warnings
Drug Details [pdf]
ANTI-BACTERIAL HAND BOARDWALK VANILLA CONE- alcohol gel
Bath & Body Works, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
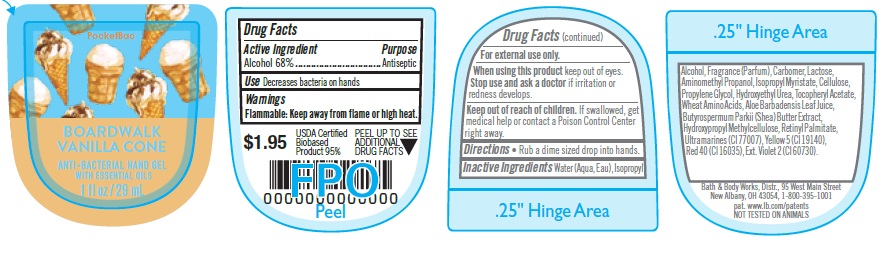
DRUG FACTS
WARNINGS
For external use only.
When using this product keep out of eyes. Stop use and ask a doctor if irritation or redness develops.
KEEP OUT OF REACH OF CHILDREN
If swallowed, get medical help or contact a Poison Control Center right away.
INACTIVE INGREDIENTS
Water (Aqua, Eau), Isopropyl
Alcohol, Fragrance (Parfum), Carbomer, Lactose,
Aminomethyl Propanol, Isopropyl Myristate, Cellulose,
Propylene Glycol, Hydroxyethyl Urea, Tocopheryl Acetate,
Wheat Amino Acids, Aloe Barbadensis Leaf Juice,
Butyrospermum Parkii (Shea) Butter Extract,
Hydroxypropyl Methylcellulose, Retinyl Palmitate,
Ultramarines (CI 77007), Yellow 5 (CI 19140),
Red 40 (CI 16035), Ext. Violet 2 (CI 60730).
| ANTI-BACTERIAL HAND
BOARDWALK VANILLA CONE
alcohol gel |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Bath & Body Works, Inc. (878952845) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Accel Inc. | 838933430 | relabel(62670-5815) | |