TOPOTECAN injection, solution, concentrate
topotecan by
Drug Labeling and Warnings
topotecan by is a Prescription medication manufactured, distributed, or labeled by Sagent Pharmaceuticals. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use TOPOTECAN INJECTION safely and effectively. See full prescribing information for TOPOTECAN INJECTION.
TOPOTECAN injection, for intravenous use
Initial U.S. Approval: 1996WARNING: BONE MARROW SUPPRESSION
See full prescribing information for complete boxed warning.
Topotecan can cause severe myelosuppression. Administer only to patients with baseline neutrophil counts greater than or equal to 1,500 cells/mm3 and platelet count greater than or equal to 100,000/mm3. Monitor blood cell counts. (5.1)
RECENT MAJOR CHANGES
INDICATIONS AND USAGE
Topotecan injection is a topoisomerase inhibitor indicated for:
- metastatic carcinoma of the ovary after disease progression on or after initial or subsequent chemotherapy. (1.1)
- small cell lung cancer platinum-sensitive disease in patients who progressed after first-line chemotherapy. (1.2)
- combination therapy with cisplatin for Stage IV-B, recurrent, or persistent carcinoma of the cervix which is not amenable to curative treatment. (1.3)
DOSAGE AND ADMINISTRATION
- Ovarian cancer and small cell lung cancer: 1.5 mg/m2 by intravenous infusion over 30 minutes daily for 5 consecutive days, starting on Day 1 of a 21-day course. (2.1, 2.2)
- Cervical cancer: 0.75 mg/m2 by intravenous infusion over 30 minutes on Days 1, 2, and 3 repeated every 21 days in combination with cisplatin 50 mg/m2 on Day 1. (2.3)
- Renal impairment: Dose reduce topotecan injection in patients with moderate renal impairment (20 to 39 mL/min). (2.4)
DOSAGE FORMS AND STRENGTHS
Injection: 4 mg per 4 mL (1 mg per mL, topotecan free base equivalent) solution in single-dose vial (3)
CONTRAINDICATIONS
- History of severe hypersensitivity reactions to topotecan. (4)
WARNINGS AND PRECAUTIONS
- Bone marrow suppression: Administer topotecan only to patients with adequate bone marrow reserves. Monitor peripheral blood counts and adjust the dose as needed. (2.4, 5.1)
- Neutropenic enterocolitis: Fatal typhlitis can occur. (5.2)
- Interstitial lung disease (ILD): Fatal cases have occurred. Permanently discontinue for confirmed ILD. (5.3)
- Embryofetal toxicity: Can cause fetal harm. Advise women of potential risk to the fetus. (5.4, 8.1, 8.3)
- Extravasation and tissue injury: Severe cases have been reported. If extravasation occurs, immediately stop administration and institute recommended management procedures. (5.5)
ADVERSE REACTIONS
Ovarian cancer:
- The most common hematologic adverse reactions were: neutropenia (Grade 4: 80%), anemia (Grade 3/4: 41%), thrombocytopenia (Grade 4: 27%), and febrile neutropenia (23%). (6.1)
- The most common (>5%) non-hematologic adverse reactions (all grades) were: nausea, vomiting, fatigue, diarrhea, and dyspnea. (6.1)
Small cell lung cancer:
- The most common hematologic adverse reactions were: neutropenia (Grade 4: 70%), anemia (Grade 3/4: 42%), thrombocytopenia (Grade 4: 29%), and febrile neutropenia (28%). (6.1)
- The most common (>5%) non-hematologic adverse reactions (all grades) were: asthenia, dyspnea, nausea, pneumonia, abdominal pain, and fatigue. (6.1)
Cervical cancer (topotecan plus cisplatin):
- The most common hematologic adverse reactions were: neutropenia (Grade 3/4: 74%), anemia (Grade 3/4: 40%), and thrombocytopenia (Grade 3/4: 33%). (6.1)
The most common (>25% and greater than or equal to 2% more than cisplatin alone) non-hematologic adverse reactions (all grades) were: pain, vomiting, and infection/febrile neutropenia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Sagent Pharmaceuticals, Inc. at 1-866-625-1618, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Do not initiate G-CSF until 24 hours after completion of treatment with topotecan. Concomitant administration can prolong duration of neutropenia. (7)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 12/2017
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: BONE MARROW SUPPRESSION
1 INDICATIONS AND USAGE
1.1 Ovarian Cancer
1.2 Small Cell Lung Cancer
1.3 Cervical Cancer
2 DOSAGE AND ADMINISTRATION
2.1 Ovarian Cancer
2.2 Small Cell Lung Cancer
2.3 Cervical Cancer
2.4 Dose Modifications
2.5 Preparation and Intravenous Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Bone Marrow Suppression
5.2 Neutropenic Enterocolitis
5.3 Interstitial Lung Disease
5.4 Embryofetal Toxicity
5.5 Extravasation and Tissue Injury
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 G-CSF
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Ovarian Cancer
14.2 Small Cell Lung Cancer
14.3 Cervical Cancer
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: BONE MARROW SUPPRESSION
Topotecan can cause severe myelosuppression. Administer only to patients with baseline neutrophil counts of greater than or equal to 1,500 cells/mm3 and platelet counts greater than or equal to 100,000 cells/mm3. Monitor blood cell counts [see Warnings and Precautions (5.1)].
-
1 INDICATIONS AND USAGE
1.1 Ovarian Cancer
Topotecan injection, as a single agent, is indicated for the treatment of patients with metastatic carcinoma of the ovary after disease progression on or after initial or subsequent chemotherapy.
-
2 DOSAGE AND ADMINISTRATION
Verify dose using body surface area prior to dispensing. Recommended dosage should generally not exceed 4 mg intravenously [see Overdosage (10)].
2.4 Dose Modifications
Hematologic Toxicities
For single-agent use, dose reduce topotecan injection to 1.25 mg/m2 for:
- neutrophil counts of less than 500 cells/mm3, or administer granulocyte-colony stimulating factor (G-CSF) starting no sooner than 24 hours following the last dose of topotecan.
- platelet counts less than 25,000 cells/mm3 during previous cycle.
For combination use with cisplatin, dose reduce topotecan injection to 0.60 mg/m2 (and further to 0.45 mg/m2 if necessary) for:
- febrile neutropenia (defined as neutrophil counts less than 1,000 cells/mm3 with temperature of greater than or equal to 38°C (100.4°F), or administer G-CSF starting no sooner than 24 hours following the last dose of topotecan.
- platelet counts less than 25,000 cells/mm3 during previous cycle.
Renal Impairment
For single-agent use, dose reduce topotecan injection to 0.75 mg/m2 in patients with moderate renal impairment (creatinine clearance [Clcr] = 20 to 39 mL/min). Insufficient data are available in patients with severe renal impairment (Clcr less than 20 mL/min) to provide a dosage recommendation for topotecan injection [see Use in Specific Populations (8.6), Clinical Pharmacology (12.3)].
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Bone Marrow Suppression
Bone marrow suppression (primarily neutropenia) is the dose-limiting toxicity of topotecan. Neutropenia is not cumulative over time. Severe myelotoxicity has been reported when topotecan is used in combination with cisplatin [see Drug Interactions (7)].
- The following data on myelosuppression are based on an integrated safety database from 8 trials (N = 879) using topotecan injection at 1.5 mg/m2 by intravenous infusion over 30 minutes daily for 5 consecutive days, starting on Day 1 of a 21-day course in patients with ovarian cancer and small cell lung cancer and from one trial in patients with cervical cancer (N = 147) using topotecan 0.75 mg/m2 by intravenous infusion over 30 minutes daily on Days 1, 2, and 3 repeated every 21 days in combination with cisplatin 50 mg/m2 on Day 1.
Neutropenia
- Monotherapy: Grade 4 neutropenia (less than 500 cells/mm3) occurred in 78% of patients, with a median duration of 7 days and was most common during Course 1 of treatment (58% of patients). Grade 4 neutropenia associated with infection occurred in 13% of patients and febrile neutropenia occurred in 5% of patients. Sepsis occurred in 4% of patients and was fatal in 1% of patients. Pancytopenia has been reported.
- Combination with cisplatin: Grade 4 neutropenia occurred in 48% of patients.
Thrombocytopenia
- Monotherapy: Grade 4 thrombocytopenia (less than 25,000/mm3) occurred in 27% of patients, with a median duration of 5 days.
- Combination with cisplatin: Grade 4 thrombocytopenia occurred in 7% of patients.
Anemia
- Monotherapy: Grade 3 or 4 anemia (less than 8 g/dL) occurred in 37% of patients.
- Combination with cisplatin: Grade 3 or Grade 4 anemia occurred in 40% of patients.
Administer topotecan only to patients with a baseline neutrophil count of greater than or equal to 1,500 cells/mm3 and a platelet count greater than or equal to 100,000/mm3. Monitor peripheral blood counts frequently during treatment with topotecan. Refer to Section 2.4 for dose modification guidelines for hematological toxicities in subsequent courses. Do not treat patients with subsequent courses of topotecan until neutrophils recover to greater than 1,000 cells/mm3, platelets recover to greater than 100,000 cells/mm3, and hemoglobin levels recover to 9 g/dL (with transfusion if necessary).
5.2 Neutropenic Enterocolitis
Topotecan can cause fatal typhlitis (neutropenic enterocolitis). Consider the possibility of typhlitis in patients presenting with fever, neutropenia, and abdominal pain.
5.3 Interstitial Lung Disease
Interstitial lung disease (ILD), including fatalities, has occurred with topotecan. Underlying risk factors include history of ILD, pulmonary fibrosis, lung cancer, thoracic radiation, and use of pneumotoxic drugs and/or colony stimulating factors. Monitor patients for pulmonary symptoms indicative of ILD (e.g., cough, fever, dyspnea, and/or hypoxia), and discontinue topotecan if a new diagnosis of ILD is confirmed.
5.4 Embryofetal Toxicity
Based on animal data, topotecan can cause fetal harm when administered to a pregnant woman. Topotecan caused embryolethality, fetotoxicity, and teratogenicity in rats and rabbits when administered during organogenesis. Advise females of reproductive potential to use effective contraception during treatment and for at least 1 month after the last dose of topotecan. Advise women of the potential risk to a fetus [see Use in Specific Populations (8.1, 8.3)].
5.5 Extravasation and Tissue Injury
Extravasation with topotecan has been observed; severe cases have been reported. If signs or symptoms of extravasation occur, immediately stop administration of topotecan and institute recommended management procedures [see Adverse Reactions (6)].
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described below and elsewhere in the labeling:
- Bone Marrow Suppression [see Warnings and Precautions (5.1)]
- Neutropenic Enterocolitis [see Warnings and Precautions (5.2)]
- Interstitial Lung Disease [see Warnings and Precautions (5.3)]
- Extravasation and Tissue Injury [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Ovarian Cancer
Table 1 shows the Grade 3/4 hematologic and major non-hematologic adverse reactions in the topotecan/paclitaxel comparator trial in ovarian cancer.
Table 1. Adverse Reactions Experienced by ≥5% of Ovarian Cancer Patients Randomized to Receive Topotecan or Paclitaxel 1 Death related to sepsis occurred in 2% of patients receiving topotecan and 0% of patients receiving paclitaxel.
2 Pain includes body pain, skeletal pain, and back pain.
Adverse Reaction Topotecan (n = 112) Paclitaxel (n = 114) Hematologic Grade 3/4 % % Grade 4 neutropenia (<500 cells/mm3) 80 21 Grade 3/4 anemia (Hgb <8 g/dL) 41 6 Grade 4 thrombocytopenia (<25,000 plts/mm3) 27 3 Febrile neutropenia 23 4 Non-hematologic Grade 3/4 % % Infections and infestations Sepsis1 5 2 Respiratory, thoracic, and mediastinal disorders Dyspnea 6 5 Gastrointestinal disorders Abdominal pain 5 4 Constipation 5 0 Diarrhea 6 1 Intestinal obstruction 5 4 Nausea 10 2 Vomiting 10 3 General disorders and administrative site conditions Fatigue 7 6 Asthenia 5 3 Pain2 5 7 Small Cell Lung Cancer
Table 2 shows the Grade 3/4 hematologic and major non-hematologic adverse reactions in the topotecan/CAV (cyclophosphamide-doxorubicin-vincristine) comparator trial in small cell lung cancer.
Table 2. Adverse Reactions Experienced by ≥5% of Small Cell Lung Cancer Patients Randomized to Receive Topotecan or CAV 1 Death related to sepsis occurred in 3% of patients receiving topotecan and 1% of patients receiving CAV.
2 Pain includes body pain, skeletal pain, and back pain.
Adverse ReactionTopotecan
(n = 107)CAV
(n = 104)Hematologic Grade 3/4 % % Grade 4 neutropenia (<500 cells/mm3) 70 72 Grade 3/4 anemia (Hgb <8 g/dL) 42 20 Grade 4 thrombocytopenia (<25,000 plts/mm3) 29 5 Febrile neutropenia 28 26 Non-hematologic Grade 3/4 % % Infections and infestations Sepsis1 5 5 Respiratory, thoracic, and mediastinal disorders Dyspnea 9 14 Pneumonia 8 6 Gastrointestinal disorders Abdominal pain 6 4 Nausea 8 6 General disorders and administrative site conditions Fatigue 6 10 Asthenia 9 7 Pain2 5 7 Hepatobiliary Disorders in Ovarian and Small Cell Lung Cancer Patients Receiving Topotecan: Based on the combined experience of 453 patients with metastatic ovarian carcinoma, and 426 patients with small cell lung cancer treated with topotecan, Grade 1 transient elevations in hepatic enzymes occurred in 8% of patients. Grade 3/4 elevations occurred in 4%. Grade 3/4 elevated bilirubin occurred in less than 2% of patients.
Cervical Cancer
In the comparative trial with topotecan plus cisplatin versus cisplatin in patients with cervical cancer, the most common dose-limiting adverse reaction was myelosuppression. Table 3 shows the hematologic and non-hematologic adverse reactions in patients with cervical cancer.
Table 3. Adverse Reactions Experienced by ≥5% of Patients with Cervical Cancer Randomized to Receive Topotecan plus Cisplatin or Cisplatin Monotherapy (Between-Arm Difference ≥2%)1 1 Includes patients who were eligible and treated.
2 Data were collected using NCI Common Toxicity Criteria, v. 2.0
3 Grades 1 through 4 only. There were 3 patients who experienced deaths with investigator-designated attribution. The first patient experienced a Grade 5 hemorrhage in which the drug-related thrombocytopenia aggravated the event. A second patient experienced bowel obstruction, cardiac arrest, pleural effusion, and respiratory failure which were not treatment-related but probably aggravated by treatment. A third patient experienced a pulmonary embolism and adult respiratory distress syndrome; the latter was indirectly treatment-related.
4 Constitutional includes fatigue (lethargy, malaise, asthenia), fever (in the absence of neutropenia), rigors, chills, sweating, and weight gain or loss.
5 Pain includes abdominal pain or cramping, arthralgia, bone pain, chest pain (non-cardiac and non-pleuritic), dysmenorrhea, dyspareunia, earache, headache, hepatic pain, myalgia, neuropathic pain, pain due to radiation, pelvic pain, pleuritic pain, rectal or perirectal pain, and tumor pain.
6 High-level terms were included if the between-arm difference was ≥10%.
Adverse ReactionTopotecan
plus Cisplatin
(n = 140)
%
Cisplatin
(n = 144)
%Hematologic Neutropenia Grade 3 (<1,000 to 500 cells/mm3) 26 1 Grade 4 (<500 cells/mm3) 48 1 Anemia Grade 3 (Hgb <8 to 6.5 g/dL) 34 19 Grade 4 (Hgb <6.5 g/dL) 6 3 Thrombocytopenia Grade 3 (<50,000 to 10,000 cells/mm3) 26 3 Grade 4 (<10,000 cells/mm3) 7 0 Non-hematologic2,3 General disorders and administrative site conditions Constitutional4 69 62 Pain5 59 50 Gastrointestinal disorders Vomiting 40 37 Stomatitis-pharyngitis 6 0 Other 63 56 Dermatology6 48 20 Infection-febrile neutropenia6 28 18 Cardiovascular6 25 15 6.2 Postmarketing Experience
The following reactions have been identified during postmarketing use of topotecan. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These reactions have been chosen for inclusion due to a combination of their seriousness, frequency of reporting, or potential causal connection to topotecan.
Blood and Lymphatic System Disorders
Severe bleeding (in association with thrombocytopenia) [see Warnings and Precautions (5.1)].
Immune System Disorders
Allergic manifestations, anaphylactoid reactions.
Gastrointestinal Disorders
Abdominal pain potentially associated with neutropenic enterocolitis [see Warnings and Precautions (5.2)].
Pulmonary Disorders
Interstitial lung disease [see Warnings and Precautions (5.3)].
Skin and Subcutaneous Tissue Disorders
Angioedema, severe dermatitis, severe pruritus.
General Disorders and Administration Site Conditions
Extravasation [see Warnings and Precautions (5.5)].
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on animal data, topotecan can cause fetal harm when administered to a pregnant woman. Topotecan caused embryolethality, fetotoxicity, and teratogenicity in rats and rabbits when administered during organogenesis at doses similar to the clinical dose [see Data]. There are no available human data informing the drug-associated risk. Advise pregnant women of the potential risk to a fetus. The background risk of major birth defects and miscarriage for the indicated populations are unknown; however, the background risk in the US general population of major birth defects is 2% to 4% and of miscarriage is 15% to 20% of clinically recognized pregnancies.
Data
Animal Data: In rabbits, a dose of 0.10 mg/kg/day (about equal to the clinical dose on a mg/m2 basis) given on Days 6 through 20 of gestation caused maternal toxicity, embryolethality, and reduced fetal body weight. In the rat, a dose of 0.23 mg/kg/day (about equal to the clinical dose on a mg/m2 basis) given for 14 days before mating through gestation Day 6 caused fetal resorption, microphthalmia, pre-implant loss, and mild maternal toxicity. Administration of an intravenous dose of 0.10 mg/kg/day (about half the clinical dose on a mg/m2 basis) given to rats on Days 6 through 17 of gestation caused an increase in post-implantation mortality. This dose also caused an increase in total fetal malformations. The most frequent malformations were of the eye (microphthalmia, anophthalmia, rosette formation of the retina, coloboma of the retina, ectopic orbit), brain (dilated lateral and third ventricles), skull, and vertebrae.
8.2 Lactation
Risk Summary
It is not known whether this drug is present in human milk; however, topotecan is excreted in rat milk at high concentrations [see Data]. Because many drugs are present in human milk and because of the potential for serious adverse reactions in nursing infants with topotecan, advise nursing mothers to discontinue breastfeeding during treatment with topotecan.
8.3 Females and Males of Reproductive Potential
Contraception
Females: Advise female patients of reproductive potential to use effective contraception during treatment with topotecan and for one month after the last dose. Advise females to contact their healthcare provider if they become pregnant, or if pregnancy is suspected, while taking topotecan [see Use in Specific Populations (8.1)].
Males: Topotecan may damage spermatozoa, resulting in possible genetic and fetal abnormalities. Advise males with a female sexual partner of reproductive potential to use effective contraception during and for three months after treatment with topotecan [see Nonclinical Toxicology (13.1)].
Females: Topotecan may have both acute and long-term effects on fertility [see Nonclinical Toxicology (13.1)].
Males: Effects on spermatogenesis have been observed in animals administered topotecan. Advise males of the potential risk for impaired fertility and to seek counseling on fertility and family planning options prior to starting treatment [see Nonclinical Toxicology (13.1)].
8.5 Geriatric Use
Of the 879 patients with metastatic ovarian cancer or small cell lung cancer in clinical trials of topotecan, 32% (n = 281) were aged 65 years and older, while 3.8% (n = 33) were aged 75 years and older. Of the 140 patients with Stage IV-B, relapsed, or refractory cervical cancer in clinical trials of topotecan who received topotecan plus cisplatin in the randomized clinical trial, 6% (n = 9) were aged 65 years and older, while 3% (n = 4) were aged 75 years and older.
No overall differences in effectiveness or safety were observed between these patients and younger adult patients, and other reported clinical experience has not identified differences in responses between the elderly and younger adult patients.
8.6 Renal Impairment
The systemic exposure to both topotecan lactone and total topotecan increased in patients with moderate renal impairment (Clcr = 20 to 39 mL/min) compared with patients with normal renal function (Clcr greater than 60 mL/min). Reduce the dose of topotecan in patients with moderate renal impairment (Clcr = 20 to 39 mL/min). No dosage adjustment of topotecan is recommended for patients with mild renal impairment (Clcr = 40 to 60 mL/min) [see Dosage and Administration (2.4), Clinical Pharmacology (12.3)].
Insufficient data are available in patients with severe renal impairment (Clcr less than 20 mL/min) to provide a dosage recommendation for topotecan.
-
10 OVERDOSAGE
Overdoses (up to 10-fold of the prescribed dose) occurred in patients treated with intravenous topotecan. The primary complication of overdosage is bone marrow suppression. The observed signs and symptoms of overdose are consistent with the known adverse reactions associated with topotecan for intravenous use [see Adverse Reactions (6.1, 6.2)]. In addition, elevated hepatic enzymes and mucositis have been reported following overdose. One patient received a single dose of 40 mg/m2 of intravenous topotecan and developed gastrointestinal toxicity, skin toxicity, and myelosuppression leading to septic shock. Another patient received a single dose of 35 mg/m2 and experienced severe, reversible neutropenia.
There is no known antidote for overdosage with topotecan. If an overdose is suspected, monitor the patient closely for bone marrow suppression and institute supportive-care measures (such as the prophylactic use of G-CSF and antibiotic therapy) as appropriate.
-
11 DESCRIPTION
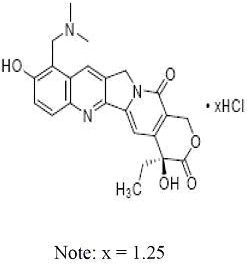
Topotecan is a semi-synthetic derivative of camptothecin and is an anti-tumor drug with topoisomerase I-inhibitory activity. The chemical name for topotecan hydrochloride is (S)-10-[(dimethylamino)methyl]-4-ethyl-4,9-dihydroxy-1H-pyrano[3',4':6,7] indolizino [1,2-b]quinoline-3,14-(4H,12H)-dione 1.25 hydrochloride. It has the molecular formula C23H23N3O5xHCl (x = 1.25) and a molecular weight of 467.02. It is soluble in water and melts with decomposition at 213°C to 218°C.
As formulated in topotecan injection, topotecan hydrochloride has the following structural formula:
Topotecan injection is supplied as a sterile, non-pyrogenic, clear, light yellow to greenish solution in single-dose vials at a topotecan free base concentration of 4 mg per 4 mL (1 mg per mL).
Each mL contains topotecan hydrochloride equivalent to 1 mg of topotecan free base, 12 mg of mannitol, USP, and 5 mg of tartaric acid, NF. It may also contain hydrochloric acid and sodium hydroxide to adjust the pH. The solution pH ranges from 2.0 to 2.5.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Topoisomerase I relieves torsional strain in DNA by inducing reversible single-strand breaks. Topotecan binds to the topoisomerase I-DNA complex and prevents re-ligation of these single-strand breaks. The cytotoxicity of topotecan is thought to be due to double-strand DNA damage produced during DNA synthesis, when replication enzymes interact with the ternary complex formed by topotecan, topoisomerase I, and DNA. Mammalian cells cannot efficiently repair these double-strand breaks.
12.3 Pharmacokinetics
Following administration of topotecan at doses of 0.5 to 1.5 mg/m2 administered as a 30-minute infusion to cancer patients, topotecan exhibited multiexponential pharmacokinetics with a terminal half-life of 2 to 3 hours. Total exposure (AUC) is approximately dose-proportional.
Metabolism
Topotecan undergoes a reversible pH-dependent hydrolysis of its lactone moiety; it is the lactone form that is pharmacologically active. At pH ≤4, the lactone is exclusively present, whereas the ring-opened hydroxy-acid form predominates at physiologic pH. In vitro studies in human liver microsomes indicate topotecan is metabolized to an N-demethylated metabolite. The mean metabolite:parent AUC ratio was about 3% for total topotecan and topotecan lactone following IV administration.
Excretion
Renal clearance is the primary route of topotecan elimination.
In a mass balance/excretion trial in 4 patients with solid tumors, the overall recovery of total topotecan and its N-desmethyl metabolite in urine and feces over 9 days averaged 73.4% ± 2.3% of the administered IV dose. Mean values of 50.8% ± 2.9% as total topotecan and 3.1% ± 1% as N-desmethyl topotecan were excreted in the urine following IV administration. Fecal elimination of total topotecan accounted for 17.9% ± 3.6% while fecal elimination of N-desmethyl topotecan was 1.7% ± 0.6%. An O-glucuronidation metabolite of topotecan and N-desmethyl topotecan has been identified in the urine.
Specific Populations
Gender: Plasma clearance of topotecan lactone in male patients was approximately 24% higher than that in female patients, largely reflecting difference in body size.
Age: Population pharmacokinetic analysis in female patients did not identify age as a significant factor. Decreased renal clearance, which is common in the elderly, is a more important determinant of topotecan clearance [see Dosage and Administration (2.4), Use in Specific Populations (8.5)].
Renal Impairment: In patients with mild renal impairment (Clcr = 40 to 60 mL/min), plasma clearance of topotecan lactone was decreased by 33% compared with patients with normal renal function (Clcr greater than 60 mL/min). In patients with moderate renal impairment (Clcr = 20 to 39 mL/min), plasma clearance of topotecan lactone was reduced by 65% compared with patients with normal renal function. Dosage adjustment is recommended for patients with moderate renal impairment. No dosage adjustment is required in patients with mild renal impairment [see Dosage and Administration (2.4), Use in Specific Populations (8.6)].
Drug Interactions
Effects of Topotecan on Drug-Metabolizing Enzymes: In vitro inhibition studies using marker substrates for human P450 CYP1A2, CYP2A6, CYP2C8/9, CYP2C19, CYP2D6, CYP2E, CYP3A, or CYP4A or dihydropyrimidine dehydrogenase indicate that the activities of these enzymes were not altered by topotecan.
Cisplatin: Administration of cisplatin (60 or 75 mg/m2 on Day 1) before topotecan (0.75 mg/m2/day on Days 1 to 5) in 9 patients with ovarian cancer had no significant effect on the Cmax and AUC of total topotecan.
Topotecan (0.3 mg/m2 IV daily on Days 2 to 6) had no effect on the pharmacokinetics of free platinum in 15 patients with ovarian cancer who were administered cisplatin 50 mg/m2 (n = 9) or 75 mg/m2 (n = 6) on Day 2 after paclitaxel 110 mg/m2 on Day 1. Topotecan (0.75 mg/m2 IV daily on Days 1 to 5) had no effect on dose-normalized (60 mg/m2) Cmax values of free platinum in 13 patients with ovarian cancer who were administered 60 mg/m2 (n = 10) or 75 mg/m2 (n = 3) cisplatin on Day 1.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity testing of topotecan has not been performed. Topotecan is known to be genotoxic to mammalian cells and is a probable carcinogen. Topotecan was mutagenic to L5178Y mouse lymphoma cells and clastogenic to cultured human lymphocytes with and without metabolic activation. It was also clastogenic to mouse bone marrow. Topotecan did not cause mutations in bacterial cells.
Topotecan given to female rats prior to mating at a dose of 1.4 mg/m2 IV (about equal to the clinical dose on a mg/m2 basis) caused superovulation possibly related to inhibition of follicular atresia. This dose given to pregnant female rats also caused increased pre-implantation loss. Studies in dogs given 0.4 mg/m2 IV (about 0.25 times the clinical dose on a mg/m2 basis) of topotecan daily for a month suggest that treatment may cause an increase in the incidence of multinucleated spermatogonial giant cells in the testes. Topotecan may impair fertility in women and men.
-
14 CLINICAL STUDIES
14.1 Ovarian Cancer
Another formulation of topotecan was studied in 2 clinical trials of 223 patients given topotecan with metastatic ovarian carcinoma. All patients had disease that had recurred on, or was unresponsive to, a platinum-containing regimen. Patients in these 2 trials received an initial dose of 1.5 mg/m2 given by intravenous infusion over 30 minutes for 5 consecutive days, starting on Day 1 of a 21-day course.
One trial was a randomized trial of 112 patients treated with topotecan (1.5 mg/m2/day × 5 days starting on Day 1 of a 21-day course) and 114 patients treated with paclitaxel (175 mg/m2 over 3 hours on Day 1 of a 21-day course). All patients had recurrent ovarian cancer after a platinum-containing regimen or had not responded to at least 1 prior platinum-containing regimen. Patients who did not respond to the trial therapy, or who progressed, could be given the alternative treatment. The efficacy outcome measures were response rate, response duration, and time to progression.
The results of the trial did not show statistically significant improvements in response rates, response duration, time to progression, and overall survival as shown in Table 4.
Table 4. Efficacy of Topotecan versus Paclitaxel in Ovarian Cancer HR = hazard-ratio; CI = confidence interval.
1 The calculation for duration of response was based on the interval between first response and time to progression.
ParameterTopotecan
(n = 112)Paclitaxel
(n = 114)Overall response rate (95% CI) 21% (13% to 28%) 14% (8% to 20%) Complete response rate 5% 3% Partial response rate 16% 11% Response duration1 (months) Median (95% CI) 6 (5.1 to 7.6) 5 (3.7 to 7.8) Time to progression (months) Median (95% CI) 4.4 (2.8 to 5.4) 3.4 (2.7 to 4.2) HR (topotecan:paclitaxel) (95% CI) 0.76 (0.57 to 1.02) Survival (months) Median (95% CI) 14.5 (10.7 to 16.5) 12.2 (9.7 to 15.8) HR (topotecan:paclitaxel) (95% CI) 0.97 (0.71 to 1.34) The median time to response was 7.6 weeks (range: 3.1 to 21.7) with topotecan compared with 6 weeks (range: 2.4 to 18.1) with paclitaxel. In the crossover phase, 8 of 61 (13%) patients who received topotecan after paclitaxel had a partial response and 5 of 49 (10%) patients who received paclitaxel after topotecan had a response (2 complete responses).
Topotecan was active in ovarian cancer patients who had developed resistance to platinum-containing therapy, defined as tumor progression while on, or tumor relapse within 6 months after completion of, a platinum-containing regimen. One complete and 6 partial responses were seen in 60 patients, for a response rate of 12%. In the same trial, there were no complete responders and 4 partial responders on the paclitaxel arm, for a response rate of 7%.
Topotecan was also studied in an open-label, non-comparative trial in 111 patients with recurrent ovarian cancer after treatment with a platinum-containing regimen, or who had not responded to 1 prior platinum-containing regimen. The response rate was 14% (95% CI: 7% to 20%). The median duration of response was 22 weeks (range: 4.6 to 41.9 weeks). The time to progression was 11.3 weeks (range: 0.7 to 72.1 weeks). The median survival was 67.9 weeks (range: 1.4 to 112.9 weeks).
14.2 Small Cell Lung Cancer
Topotecan was studied in 426 patients with recurrent or progressive small cell lung cancer in 1 randomized, comparative trial and in 3 single-arm trials.
Randomized Comparative Trial
In a randomized, comparative, Phase 3 trial, 107 patients were treated with topotecan (1.5 mg/m2/day × 5 days starting on Day 1 of a 21-day course) and 104 patients were treated with CAV (1,000 mg/m2 cyclophosphamide, 45 mg/m2 doxorubicin, 2 mg vincristine administered sequentially on Day 1 of a 21-day course). All patients were considered sensitive to first-line chemotherapy (responders who then subsequently progressed greater than or equal to 60 days after completion of first-line therapy). A total of 77% of patients treated with topotecan and 79% of patients treated with CAV received platinum/etoposide with or without other agents as first-line chemotherapy. The efficacy outcome measures were response rate and duration of response.
The results of the trial did not show statistically significant improvements in response rates, response duration, time to progression, and overall survival as shown in Table 5.
Table 5. Efficacy of Topotecan versus CAV (cyclophosphamide-doxorubicin-vincristine) in Small Cell Lung Cancer Patients Sensitive to First-Line Chemotherapy HR = hazard ratio; CI = confidence interval.
1 The calculation for duration of response was based on the interval between first response and time to progression.
ParameterTopotecan
(n = 107)CAV
(n = 104)Overall response rate (95% CI) 24% (16% to 32%) 18% (11% to 26%) Complete response rate 0% 1% Partial response rate 24% 17% Response duration1 (months) Median (95% CI) 3.3 (3 to 4.1) 3.5 (3 to 5.3) Time to progression (months) Median (95% CI) 3.1 (2.6 to 4.1) 2.8 (2.5 to 3.2) HR (topotecan:CAV) (95% CI) 0.92 (0.69 to 1.22) Survival (months) Median (95% CI) 5.8 (4.7 to 6.8) 5.7 (5 to 7) HR (topotecan:CAV) (95% CI) 1.04 (0.78 to 1.39) The time to response was similar in both arms: Topotecan median of 6 weeks (range: 2.4 to 15.7) versus CAV median 6 weeks (range: 5.1 to 18.1).
Changes on a disease-related symptom scale in patients who received topotecan or who received CAV are presented in Table 6. It should be noted that not all patients had all symptoms, nor did all patients respond to all questions. Each symptom was rated on a 4-category scale with an improvement defined as a change in 1 category from baseline sustained over 2 courses. Limitations in interpretation of the rating scale and responses preclude formal statistical analysis.
Table 6. Percentage of Patients with Symptom Improvement1: Topotecan versus CAV in Patients with Small Cell Lung Cancer 1 Defined as improvement sustained over at least 2 courses compared with baseline.
2 Number of patients with baseline and at least 1 post-baseline assessment.
Topotecan
(n = 107)CAV
(n = 104)Symptom n2 (%) n2 (%) Shortness of breath 68 (28) 61 (7) Interference with daily activity 67 (27) 63 (11) Fatigue 70 (23) 65 (9) Hoarseness 40 (33) 38 (13) Cough 69 (25) 61 (15) Insomnia 57 (33) 53 (19) Anorexia 56 (32) 57 (16) Chest pain 44 (25) 41 (17) Hemoptysis 15 (27) 12 (33) Single-Arm Trials
Topotecan was also studied in 3 open-label, non-comparative trials in a total of 319 patients with recurrent or progressive small cell lung cancer after treatment with first-line chemotherapy. In all 3 trials, patients were stratified as either sensitive (responders who then subsequently progressed greater than or equal to 90 days after completion of first-line therapy) or refractory (no response to first-line chemotherapy or who responded to first-line therapy and then progressed within 90 days of completing first-line therapy). Response rates ranged from 11% to 31% for sensitive patients and 2% to 7% for refractory patients. Median time to progression and median survival were similar in all 3 trials and the comparative trial.
14.3 Cervical Cancer
In a comparative trial, 147 eligible women were randomized to topotecan (0.75 mg/m2/day IV over 30 minutes × 3 consecutive days starting on Day 1 of a 21-day course) plus cisplatin (50 mg/m2 on Day 1) and 146 eligible women were randomized to cisplatin (50 mg/m2 IV on Day 1 of a 21-day course). All patients had histologically confirmed Stage IV-B, recurrent, or persistent carcinoma of the cervix considered not amenable to curative treatment with surgery and/or radiation. Fifty-six percent (56%) of patients treated with topotecan plus cisplatin and 56% of patients treated with cisplatin had received prior cisplatin with or without other agents as first-line chemotherapy.
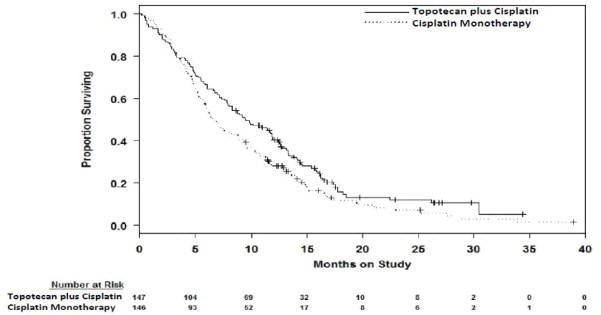
Median survival of eligible patients receiving topotecan plus cisplatin was 9.4 months (95% CI: 7.9 to 11.9) compared with 6.5 months (95% CI: 5.8 to 8.8) among patients randomized to cisplatin alone with a log rank P-value of 0.033 (significance level was 0.044 after adjusting for the interim analysis). The unadjusted hazard ratio for overall survival was 0.76 (95% CI: 0.59 to 0.98).
Figure 1. Overall Survival Curves Comparing Topotecan plus Cisplatin versus Cisplatin Monotherapy in Cervical Cancer Patients
- 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Topotecan Injection is supplied as follows:
NDC Topotecan Injection (1 mg per mL) Package Factor 25021-236-04 4 mg per 4 mL Single-Dose Vial 1 vial per carton Topotecan injection is supplied in 4 mg per 4 mL (1 mg per mL, topotecan free base equivalent) single-dose vials. Each vial contains 4 mL of the sterile, clear, light yellow to greenish solution.
16.2 Storage and Handling
Store refrigerated between 2°C and 8°C (36°F and 46°F). Handle and dispose of topotecan injection consistent with recommendations for the handling and disposal of hazardous drugs1.
Protect from light. Retain in carton until time of use.
Discard unused portion.Sterile, Nonpyrogenic, Preservative-free.
-
17 PATIENT COUNSELING INFORMATION
- Bone Marrow Suppression
Inform patients that topotecan decreases blood cell counts such as white blood cells, platelets, and red blood cells. Advise patients to notify their healthcare provider promptly for fever, other signs of infection (e.g., chills, cough, or burning pain on urination), or bleeding. Inform patients that frequent blood tests will be performed while taking topotecan to monitor for the occurrence of bone marrow suppression.
- Embryofetal Toxicity
Advise patients to contact their healthcare provider if they become pregnant, or if pregnancy is suspected during treatment with topotecan. Advise females of reproductive potential to use effective contraception during treatment and for 1 month after the last dose of topotecan. Advise males with a female sexual partner of reproductive potential to use effective contraception during treatment and for 3 months after the last dose of topotecan [see Warnings and Precautions (5.4), Use in Specific Populations (8.1, 8.3)].
- Lactation
Advise nursing mothers to discontinue breastfeeding during treatment with topotecan [see Use in Specific Populations (8.2)].
- Infertility
Advise male and female patients of the potential risk for impaired fertility and possible family planning options [see Use in Specific Populations (8.3)].
- Asthenia and Fatigue
Advise patients that topotecan may cause asthenia or fatigue. These symptoms may impair the ability to safely drive or operate machinery.
SAGENT®
Mfd. for SAGENT Pharmaceuticals
Schaumburg, IL 60195
Made in USA©2017 Sagent Pharmaceuticals, Inc.
Revised: December 2017
SAGENT Pharmaceuticals®
-
PRINCIPAL DISPLAY PANEL
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – Vial Label
NDC: 25021-236-04
Rx only
Topotecan Injection
4 mg per 4 mL (1 mg per mL)
Caution: Cytotoxic Agent
Must Dilute Before Intravenous Infusion
-
INGREDIENTS AND APPEARANCE
TOPOTECAN
topotecan injection, solution, concentrateProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 25021-236 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength topotecan hydrochloride (UNII: 956S425ZCY) (topotecan - UNII:7M7YKX2N15) topotecan 1 mg in 1 mL Inactive Ingredients Ingredient Name Strength mannitol (UNII: 3OWL53L36A) tartaric acid (UNII: W4888I119H) hydrochloric acid (UNII: QTT17582CB) sodium hydroxide (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 25021-236-04 1 in 1 CARTON 12/15/2014 01/31/2021 1 4 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022453 12/15/2014 01/31/2021 Labeler - Sagent Pharmaceuticals (796852890)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.