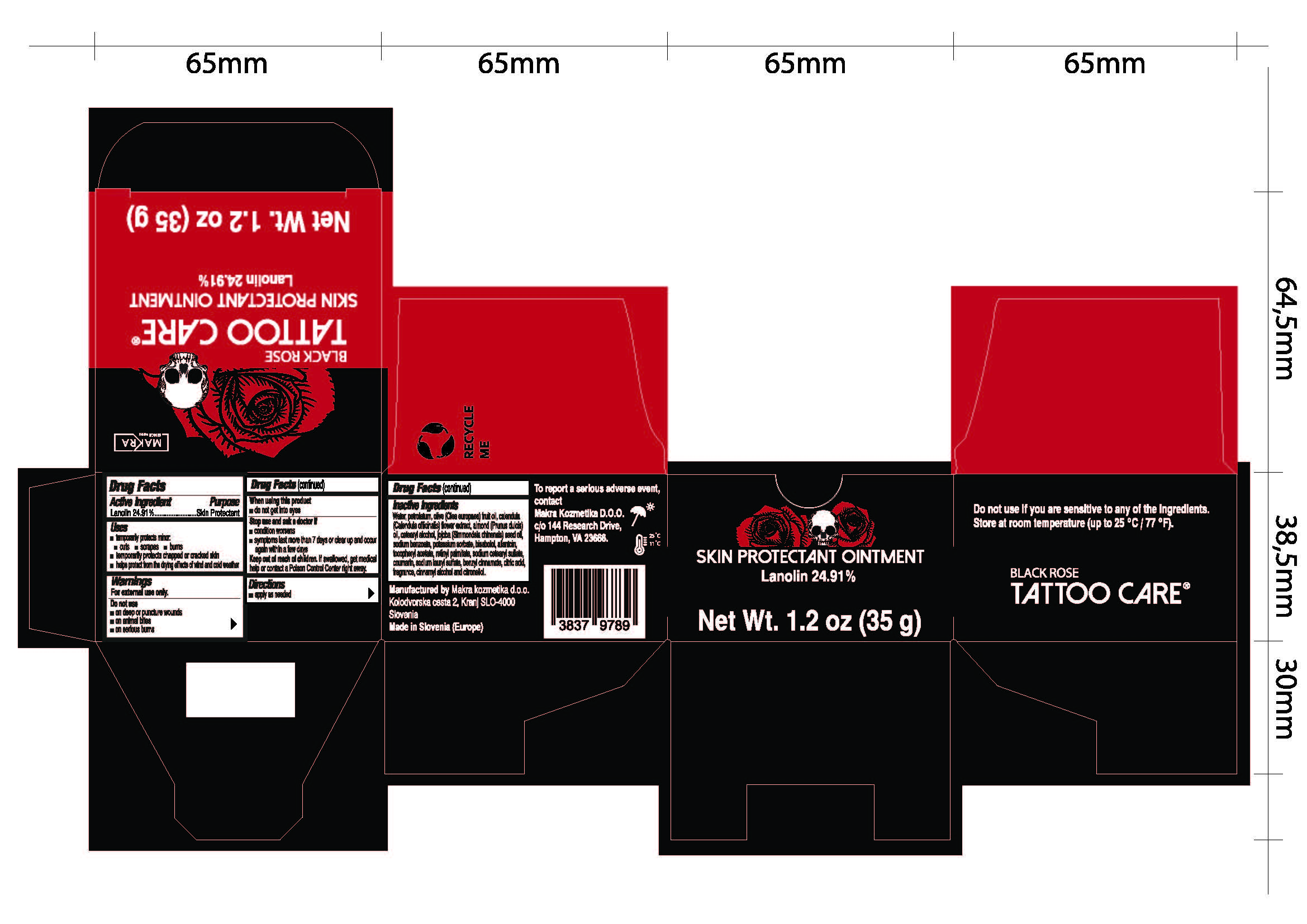
Black Rose by MAKRA KOZMETIKA D.O.O. Black Rose
Black Rose by
Drug Labeling and Warnings
Black Rose by is a Otc medication manufactured, distributed, or labeled by MAKRA KOZMETIKA D.O.O.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
BLACK ROSE- lanolin ointment
MAKRA KOZMETIKA D.O.O.
----------
Black Rose
Uses
- temporarily protects minor:
- temporarily protects chapped or cracked skin
- helps protect from the drying effects of wind and cold weather
cuts
scrapes
burns
Stop use and ask a doctor if
condition worsens
symptoms last more than 7 days or clear up and occur again within a few days
Inactive ingredients
Water, petrolatum, olive (Olea europea) fruit oil, calendula (Calendula officinalis) flower extract, almond (Prunus dulcis) oil, cetearyl alcohol, jojoba (Simmondsia chinesis) seed oil, sodium benzoate, potassium sorbate, bisabolol, allantoin, tocopheryl acetate, retinyl palmitate, sodium cetearyl sulfate, coumarin, sodium lauryl sulfate, benzyl cinnamate, citric acid, fragrance, cinnamyl alcohol and citronellol.
| BLACK ROSE
lanolin ointment |
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
| Labeler - MAKRA KOZMETIKA D.O.O. (537064093) |
| Registrant - MAKRA KOZMETIKA D.O.O. (537064093) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| MAKRA KOZMETIKA D.O.O. | 537064093 | manufacture(82806-001) | |
Revised: 10/2024
Document Id: 252aad91-df11-7e9b-e063-6294a90a0c2f
Set id: e60c2c8b-3bad-57ac-e053-2995a90a2faa
Version: 4
Effective Time: 20241023
Trademark Results [Black Rose]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BLACK ROSE 97630548 not registered Live/Pending |
Black Rose LLC 2022-10-13 |
 BLACK ROSE 97630506 not registered Live/Pending |
Black Rose LLC 2022-10-13 |
 BLACK ROSE 97033924 not registered Live/Pending |
Mensah-Cooley, Roger N 2021-09-17 |
 BLACK ROSE 90873740 not registered Live/Pending |
Anderson, Hillary A 2021-08-09 |
 BLACK ROSE 90125401 not registered Live/Pending |
Cafe Randezvous, Inc. 2020-08-19 |
 BLACK ROSE 90039584 not registered Live/Pending |
Bruce H. Turner 2020-07-07 |
 BLACK ROSE 90039574 not registered Live/Pending |
Bruce H. Turner 2020-07-07 |
 BLACK ROSE 90008458 not registered Live/Pending |
Woine INC 2020-06-18 |
 BLACK ROSE 87861682 not registered Dead/Abandoned |
YOUNG, JON VERAY 2018-04-03 |
 BLACK ROSE 86816508 4988619 Live/Registered |
Pepper Palace Inc. 2015-11-11 |
 BLACK ROSE 86470282 not registered Dead/Abandoned |
Voltron Recordz, LLC 2014-12-03 |
 BLACK ROSE 86275913 not registered Dead/Abandoned |
THE WINE GROUP LLC 2014-05-08 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.