Z-TUSS AC- chlorpheniramine maleate, codeine phosphate liquid
Z-TUSS by
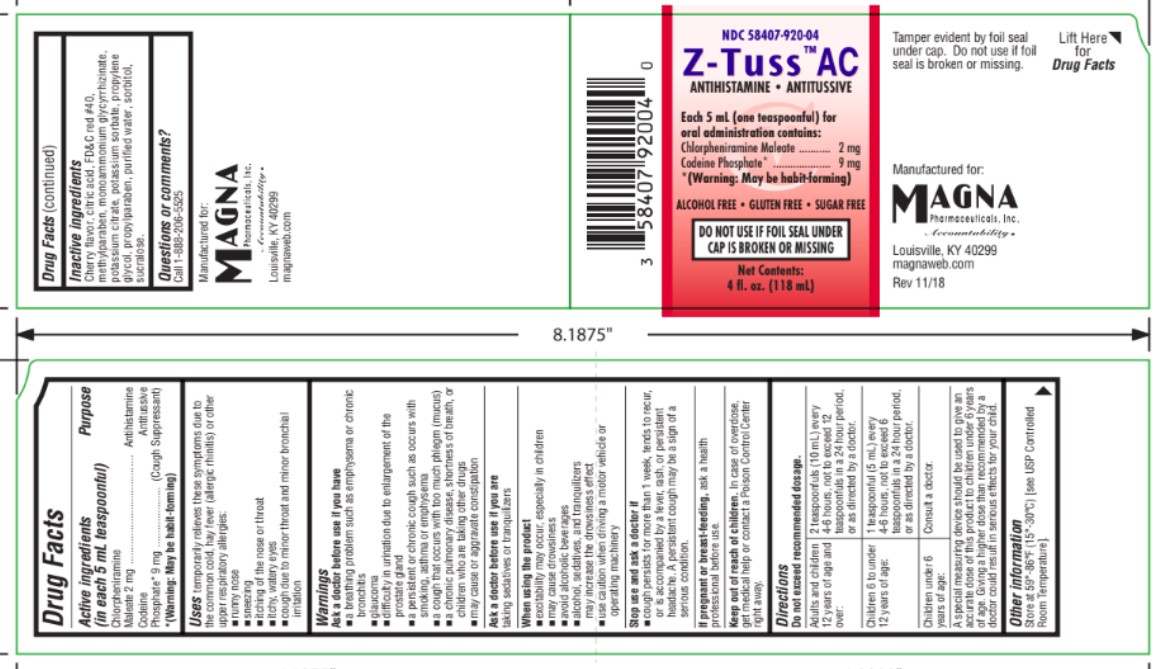
Drug Labeling and Warnings
Z-TUSS by is a Otc medication manufactured, distributed, or labeled by Magna Pharmaceuticals, Inc., Woodfield Pharmaceuticals, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Drug Facts
- Purpose
- Uses
- WARNINGS
-
ASK DOCTOR
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- difficulty in urination due to enlargement of the prostate gland
- a persistent or chronic cough such as occurs with smoking, asthma or emphysema
- a cough that occurs with too much phlegm (mucus)
- a chronic pulmonary disease, shortness of breath, or children who are taking other drugs
- ASK DOCTOR
- WHEN USING
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
Do not exceed recommended dosage.
Adults and Children
12 years of age and
over:
2 teaspoonfuls (10 mL)
every 4-6 hours, not to
exceed 12 teaspoonfuls
in a 24 hour period.
Children 6 to under
12 years of age:
1 teaspoonful (5 mL)
every 4-6 hours, not to
exceed 6 teaspoonfuls
in a 24 hour period.
Children under 6
years of age:
Consult a doctor. A special measuring device should be used to give an accurate dose of this product to children under 6 years of age. Giving a higher dose than recommended by a doctor could result in serious effects for your child.
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- QUESTIONS
- Z-Tuss AC 118mL
-
INGREDIENTS AND APPEARANCE
Z-TUSS AC
chlorpheniramine maleate, codeine phosphate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 58407-920 Route of Administration ORAL DEA Schedule CV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORPHENIRAMINE MALEATE (UNII: V1Q0O9OJ9Z) (CHLORPHENIRAMINE - UNII:3U6IO1965U) CHLORPHENIRAMINE MALEATE 2 mg in 5 mL CODEINE PHOSPHATE (UNII: GSL05Y1MN6) (CODEINE ANHYDROUS - UNII:UX6OWY2V7J) CODEINE PHOSPHATE 9 mg in 5 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SODIUM CITRATE (UNII: 1Q73Q2JULR) SORBITOL (UNII: 506T60A25R) Product Characteristics Color Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 58407-920-16 473 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/11/2011 09/01/2019 2 NDC: 58407-920-10 10 mL in 1 BOTTLE; Type 0: Not a Combination Product 09/27/2011 09/30/2013 3 NDC: 58407-920-04 118 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 01/11/2011 Labeler - Magna Pharmaceuticals, Inc. (620988360) Establishment Name Address ID/FEI Business Operations Woodfield Pharmaceuticals, LLC 079398730 manufacture(58407-920)
Trademark Results [Z-TUSS]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 Z-TUSS 74710522 2033059 Live/Registered |
MAGNA PHAMACEUTICALS, INC. 1995-08-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.