RESFLOR GOLD- florfenicol and flunixin meglumine injection
Resflor Gold by
Drug Labeling and Warnings
Resflor Gold by is a Animal medication manufactured, distributed, or labeled by Merck Sharp & Dohme Corp.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- CAUTION
-
DESCRIPTION
RESFLOR GOLD® is an injectable solution of the synthetic antibiotic florfenicol and the non-steroidal anti-inflammatory drug (NSAID) flunixin. Each milliliter of sterile RESFLOR GOLD® contains 300 mg florfenicol, 16.5 mg flunixin as flunixin meglumine, 300 mg 2-pyrrolidone, 35 mg malic acid, and triacetin qs.
- INDICATION
-
DOSAGE AND ADMINISTRATION
RESFLOR GOLD® should be administered once by subcutaneous injection at a dose rate of 40 mg florfenicol/kg body weight and 2.2 mg flunixin/kg body weight (6 mL/100 lb). Do not administer more than 10 mL at each site. The injection should be given only in the neck. Injection sites other than the neck have not been evaluated. For the 500 mL vial, do not puncture the stopper more than 20 times.
- CONTRAINDICATIONS
-
WARNINGS
NOT FOR HUMAN USE. KEEP OUT OF REACH OF CHILDREN. This product contains material that can be irritating to skin and eyes. Avoid direct contact with skin, eyes, and clothing. In case of accidental eye exposure, flush with water for 15 minutes. In case of accidental skin exposure, wash with soap and water. Remove contaminated clothing. Consult a physician if irritation persists. Accidental injection of this product may cause local irritation. Consult a physician immediately. The Material Safety Data Sheet (MSDS) contains more detailed occupational safety information.
For customer service or to obtain a copy of the MSDS, call 1-800-211-3573. For technical assistance or to report suspected adverse reactions, call 1-800-219-9286.
-
PRECAUTIONS
As a class, cyclo-oxygenase inhibitory NSAIDs may be associated with gastrointestinal, renal, and hepatic toxicity. Sensitivity to drug-associated adverse events varies with the individual patient. Patients at greatest risk for adverse events are those that are dehydrated, on diuretic therapy, or those with existing renal, cardiovascular, and/or hepatic dysfunction. Concurrent administration of potentially nephrotoxic drugs should be carefully monitored. NSAIDs may inhibit the prostaglandins that maintain normal homeostatic function. Such anti-prostaglandin effects may result in clinically significant disease in patients with underlying or pre-existing disease that have not been previously diagnosed. Since many NSAIDs possess the potential to produce gastrointestinal ulceration, concominant use of RESFLOR GOLD® with other anti-inflammatory drugs, such as NSAIDs or corticosteroids, should be avoided or closely monitored.
Flunixin is a cyclo-oxygenase inhibitory NSAID, and as with others in this class, adverse effects may occur with its use. The most frequently reported adverse effects have been gastrointestinal signs. Events involving suspected renal, hematologic, neurologic, dermatologic, and hepatic effects have also been reported for other drugs in this class.
Not for use in animals intended for breeding purposes. The effects of florfenicol on bovine reproductive performance, pregnancy, and lactation have not been determined. Toxicity studies in dogs, rats, and mice have associated the use of florfenicol with testicular degeneration and atrophy. NSAIDs are known to have potential effects on both parturition and the estrous cycle. There may be a delay in the onset of estrus if flunixin is administered during the prostaglandin phase of the estrous cycle. The effects of flunixin on imminent parturition have not been evaluated in a controlled study. NSAIDs are known to have the potential to delay parturition through a tocolytic effect.
RESFLOR GOLD®, when administered as directed, may induce a transient reaction at the site of injection and underlying tissues that may result in trim loss of edible tissue at slaughter.
-
RESIDUE WARNINGS
Animals intended for human consumption must not be slaughtered within 38 days of treatment. This product is not approved for use in female dairy cattle 20 months of age or older, including dry dairy cows. Use in these cattle may cause drug residues in milk and/or in calves born to these cows. A withdrawal period has not been established in pre-ruminating calves. Do not use in calves to be processed for veal.
-
ADVERSE REACTIONS
Transient inappetence, diarrhea, decreased water consumption, and injection site swelling have been associated with the use of florfenicol in cattle. In addition, anaphylaxis and collapse have been reported post-approval with the use of another formulation of florfenicol in cattle. In cattle, rare instances of anaphylactic-like reactions, some of which have been fatal, have been reported, primarily following intravenous use of flunixin meglumine.
-
CLINICAL PHARMACOLOGY
The pharmacokinetics (PK) of florfenicol (Table 1) and flunixin (Table 2) after subcutaneous injection of RESFLOR GOLD® is described below:
Table 1. Mean (n=28) pharmacokinetic parameters for florfenicol in cattle after a single subcutaneous administration of RESFLOR GOLD (florfenicol dose of 40 mg/kg BW). Mean Florfenicol PK parameters in Cattle PK
ParameterAUC 0-t *
(ng*hr/mL)AUC0-inf †
(ng*hr/mL)Cmax‡
(ng/mL)Tmax§ (hr) T½¶ (hr) MRT0-inf #
(hr)Mean 242527 247577 11151 6.25 28.5 27.3 SDß 42741 41391 4194 3.87 9.91 11.6 Table 2. Mean (n=28) pharmacokinetic parameters for flunixin in cattle after a single subcutaneous administration of RESFLOR GOLD (flunixin dose of 2.2 mg/kg BW). Mean Flunixin PK parameters in Cattle PK
ParameterAUC 0-t *
(ng*hr/mL)AUC0-inf †
(ng*hr/mL)Cmax‡
(ng/mL)Tmax§ (hr) T½¶ (hr) MRT0-inf #
(hr)- * AUC 0-t = Area under the plasma-concentration-time curve (AUC) from time zero to the last quantifiable concentrations
- † AUC0-inf = AUC from time zero to infinity
- ‡ Cmax = Maximum plasma concentration
- § Tmax = Time at which Cmax was observed
- ¶ T½ = Terminal elimination half-life
- # MRT0-inf = Mean residence time from time zero to infinity
- Þ n=27
- ß SD = Standard deviation
Mean 13370 14448Þ 1913 1.14 9.5Þ 11.4 SD ß 4964 5116 791 0.97 3.27 4.41 -
MICROBIOLOGY
Florfenicol is a synthetic, broad-spectrum antibiotic active against many Gram-negative and Gram-positive bacteria isolated from domestic animals. It acts by binding to the 50S ribosomal subunit and inhibiting bacterial protein synthesis. Florfenicol is generally considered a bacteriostatic drug, but exhibits bactericidal activity against certain bacterial species. In vitro studies demonstrate that florfenicol is active against the BRD pathogens M. haemolytica, P. multocida, and H. somni, and M. bovis that florfenicol exhibits bactericidal activity against strains of M. haemolytica and H. somni.
The minimum inhibitory concentrations (MICs) of florfenicol were determined for BRD isolates obtained from calves enrolled in BRD field studies in the U.S. in 2006 using methods recommended by the Clinical and Laboratory Standards Institute (M31-A2). MICs for M. bovis isolates were determined by an accepted method using Hayflick Broth with Alamar Blue (HBAN) medium under appropriate control. Isolates were obtained from pre-treatment nasal swabs from all calves enrolled at all four sites, post-treatment nasal swabs from treatment failures in the RESFLOR GOLD and saline control treatment groups at three sites, and lung tissue from one calf that died in the saline control treatment group. The results are shown in Table 3.
Table 3. Florfenicol MIC values* of indicated pathogens isolated from cattle with naturally-ocurring BRD. Indicated pathogens Year of isolation Number of isolates MIC50†
(μg/mL)MIC90†
(μg/mL)MIC range
(μg/mL)- * The correlation between in vitro susceptibility data and clinical effectiveness is unknown.
- † The lowest MIC to encompass 50% and 90% of the most susceptible isolates, respectively.
Mannheimia haemolytica 2006 183 1.0 1.0 0.5 to 32 Pasteurella multocida 2006 139 0.5 0.5 ≤ 0.125 to 16 Histophilus somni 2006 84 ≤ 0.125 ≤ 0.125 ≤ 0.125 to 0.25 Mycoplasma bovis 2006 60 1.0 1.0 0.5 to 1.0 -
EFFECTIVENESS
In a multi-site field study, calves with naturally-occurring BRD were treated with RESFLOR GOLD®, Nuflor Gold® (NADA 141-265), or saline. A treatment success was defined as a calf with normal respiration to mild respiratory distress, normal attitude to mildly depressed, and a rectal temperature < 104.0 °F on Day 11. The treatment success rate for BRD for the RESFLOR GOLD® treatment group (68.4%) was statistically significantly greater (p = 0.0255) compared to the saline control treatment group (42.9%). RESFLOR GOLD® was non-inferior to Nuflor Gold® for the treatment of BRD, with a one-sided 95% lower confidence bound for the difference between the two treatments equal to -13.2%.
In the same study, the change in rectal temperature from pre-treatment to six hours post-treatment was evaluated to determine the effectiveness of RESFLOR GOLD® for the control of BRD-associated pyrexia. The proportion of calves whose rectal temperatures decreased by ≥ 2.0 °F from pre-treatment to six hours post-treatment was statistically significantly greater (p = 0.0019) in the RESFLOR GOLD® treatment group compared to the saline control treatment group. The mean decrease in rectal temperature from pre-treatment to six hours post-treatment was statistically significantly greater in the RESFLOR GOLD® treatment group compared to the Nuflor Gold® and saline control treatment groups (p = 0.0031 and 0.0002, respectively).
The effectiveness of RESFLOR GOLD for the treatment of BRD associated with Mycoplasma bovis was demonstrated by examining the M. bovis data from cattle enrolled in the BRD treatment study described above. There were numerically more treatment successes (6 of 8 calves, 75%) than treatment failures (2 of 8 calves, 25%) in RESFLOR GOLD-treated calves that cultured positive for M. bovis pre-treatment.
-
ANIMAL SAFETY
A target animal safety study was conducted to evaluate the effects of RESFLOR GOLD® when administered to cattle subcutaneously at 1X, 3X, or 5X the labeled dose for three consecutive days (3X the labeled duration). Decreased feed and water consumption, and decreased body weights (secondary to decreased feed consumption) were observed in the 1X, 3X, and 5X groups. Injection site swellings were noted in the 1X, 3X, and 5X groups.
A separate injection site study was conducted in cattle. The study demonstrated that RESFLOR GOLD®, when administered according to the label directions, may induce a transient local reaction in the subcutaneous and underlying muscle tissue.
- STORAGE INFORMATION
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 100 mL Vial Carton
100 mL
Multiple Dose Vial
300mg/16.5mg/mLSterile
Resflor
GOLD®
Florfenicol and
Flunixin Meglumine(Florfenicol and Flunixin Meglumine)
Antimicrobial/Non-Steroidal Anti-Inflammatory DrugFor subcutaneous use in beef and
non-lactating dairy cattle only.
Not for use in female dairy cattle
20 months of age or older or in calves
to be processed for veal.CAUTION: Federal law restricts
this drug to use by or on the order of
a licensed veterinarian.NADA 141-299, Approved by FDA.
MERCK
Animal Health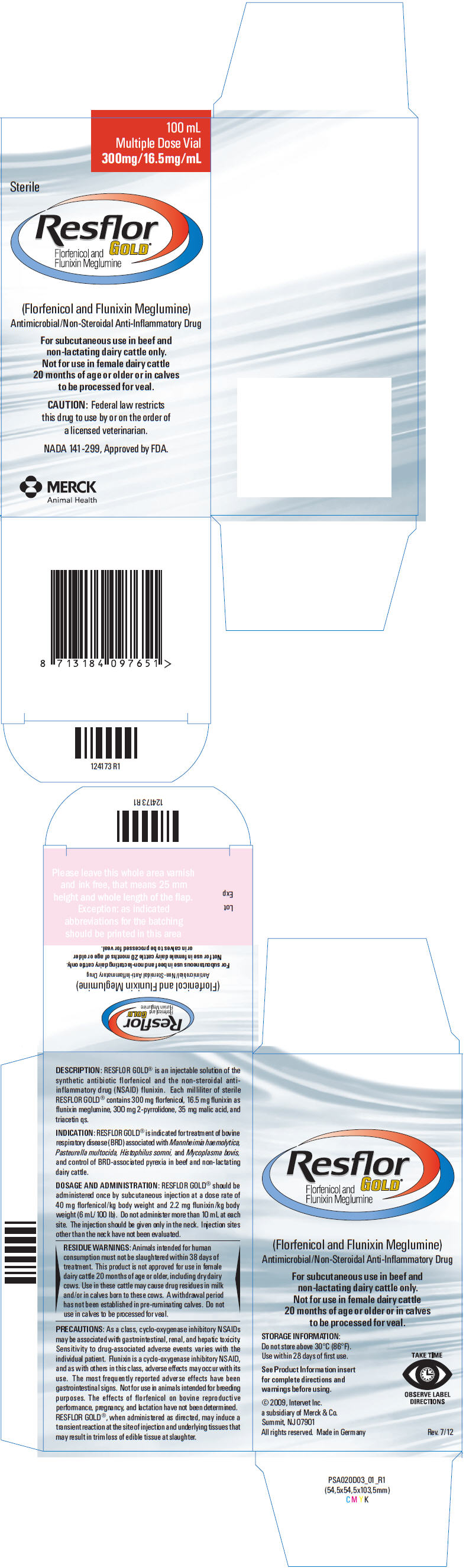
-
INGREDIENTS AND APPEARANCE
RESFLOR GOLD
florfenicol and flunixin meglumine injectionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 0061-4305 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Florfenicol (UNII: 9J97307Y1H) (Florfenicol - UNII:9J97307Y1H) Florfenicol 300 mg in 1 mL Flunixin Meglumine (UNII: 8Y3JK0JW3U) (Flunixin - UNII:356IB1O400) Flunixin Meglumine 16.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength PYRROLIDONE (UNII: KKL5D39EOL) malic acid (UNII: 817L1N4CKP) triacetin (UNII: XHX3C3X673) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0061-4305-01 1 in 1 CARTON 1 100 mL in 1 VIAL, MULTI-DOSE 2 NDC: 0061-4305-02 250 mL in 1 VIAL, MULTI-DOSE 3 NDC: 0061-4305-03 500 mL in 1 VIAL, MULTI-DOSE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141299 12/15/2009 Labeler - Merck Sharp & Dohme Corp. (001317601) Establishment Name Address ID/FEI Business Operations Vet Pharma Friesoythe GmbH 341934053 MANUFACTURE, ANALYSIS, LABEL, PACK Establishment Name Address ID/FEI Business Operations ISP Chemicals LLC 078413681 API MANUFACTURE Establishment Name Address ID/FEI Business Operations MINSHENG GROUP SHAOXING PHARMACEUTICAL CO., LTD. 544607919 API MANUFACTURE
Trademark Results [Resflor Gold]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 RESFLOR GOLD 78859528 3332442 Live/Registered |
INTERVET INC. 2006-04-12 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.