OHTUVAYRE- ensifentrine suspension
Ohtuvayre by
Drug Labeling and Warnings
Ohtuvayre by is a Prescription medication manufactured, distributed, or labeled by Verona Pharma, Inc., Hovione FarmaCiencia SA - Sete Casas, QC Pharma, The Ritedose Corporation, Quality Chemical Laboratories ("QCL"). Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use OHTUVAYRE™ safely and effectively. See full prescribing information for OHTUVAYRE.
OHTUVAYRE (ensifentrine) inhalation suspension, for oral inhalation use
Initial U.S. Approval: 2024INDICATIONS AND USAGE
OHTUVAYRE is a phosphodiesterase 3 (PDE3) inhibitor and phosphodiesterase 4 (PDE4) inhibitor indicated for the maintenance treatment of chronic obstructive pulmonary disease (COPD) in adult patients. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Inhalation suspension: 3 mg/2.5 mL aqueous suspension in unit-dose ampules. (3)
CONTRAINDICATIONS
OHTUVAYRE is contraindicated in patients with hypersensitivity to ensifentrine or any component of this product. (4)
WARNINGS AND PRECAUTIONS
- Should not use OHTUVAYRE to treat acute symptoms of bronchospasm. (5.1)
- If paradoxical bronchospasm occurs, discontinue OHTUVAYRE and institute alternative therapy. (5.2)
- An increase in psychiatric adverse reactions, including suicidality, were reported with use of OHTUVAYRE. Carefully weigh the risks and benefits of treatment with OHTUVAYRE in patients with a history of depression and/or suicidal thoughts or behavior. (5.3)
ADVERSE REACTIONS
Most common adverse reactions (incidence greater and equal to 1% and more common than placebo) include back pain, hypertension, urinary tract infection, and diarrhea. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Verona Pharma at 888-672-0371 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
Hepatic impairment: Ensifentrine exposure increases in patients with hepatic impairment. Use with caution. (8.6, 12.3)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 7/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Acute Episodes of Bronchospasm
5.2 Paradoxical Bronchospasm
5.3 Psychiatric Events Including Suicidality
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
The recommended dosage of OHTUVAYRE is 3 mg (one unit-dose ampule) twice daily, once in the morning and once in the evening, administered by oral inhalation using a standard jet nebulizer with a mouthpiece.
Administration Instructions
- Remove OHTUVAYRE unit-dose ampule from foil pouch only immediately before use. For pouches of 5 ampules, remove one ampule and place the remaining ampules back into the pouch until next use. Once the foil pouch is opened, discard ampules if not used within 14 days.
- Shake OHTUVAYRE ampule vigorously.
- Squeeze and completely empty contents of the ampule into the nebulizer cup for administration of OHTUVAYRE by oral inhalation. Discard ampule with any residual content.
- Administer OHTUVAYRE by oral inhalation using a standard jet nebulizer equipped with a mouthpiece, connected to an air compressor [see Instructions for Use].
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Acute Episodes of Bronchospasm
OHTUVAYRE should not be used for the relief of acute symptoms, i.e., as rescue therapy for the treatment of acute episodes of bronchospasm. OHTUVAYRE has not been studied in the relief of acute symptoms and extra doses of OHTUVAYRE should not be used for that purpose. The safety and effectiveness of OHTUVAYRE for relief of acute symptoms have not been established. Acute symptoms should be treated with an inhaled, short-acting bronchodilator.
5.2 Paradoxical Bronchospasm
As with other inhaled medicines, OHTUVAYRE may produce paradoxical bronchospasm, which may be life threatening. If paradoxical bronchospasm occurs following dosing with OHTUVAYRE, it should be treated immediately with an inhaled, short-acting bronchodilator. OHTUVAYRE should be discontinued immediately and alternative therapy should be instituted.
5.3 Psychiatric Events Including Suicidality
Treatment with OHTUVAYRE is associated with an increase in psychiatric adverse reactions. Psychiatric events including suicide-related adverse reactions were reported in clinical studies in patients who received OHTUVAYRE. One patient who received OHTUVAYRE in the pooled 24-week safety population [see Adverse Reactions (6.1)] experienced a suicide-related adverse reaction (suicide attempt), and in another controlled study, one patient who received ensifentrine experienced a suicide-related adverse reaction (suicide). Additionally, the most commonly reported psychiatric adverse reactions in the pooled 24-week safety population were insomnia (6 patients [0.6%] OHTUVAYRE 3 mg; 2 patients [0.3%] placebo), and anxiety (2 patients [0.2%] OHTUVAYRE 3 mg; 1 patient [0.2%] placebo). Depression-related reactions including depression, major depression, and adjustment disorder with depressed mood occurred in 4 patients [0.4%] receiving OHTUVAYRE and no patients receiving placebo.
Before initiating treatment with OHTUVAYRE, healthcare providers should carefully weigh the risk and benefits of treatment with OHTUVAYRE in patients with a history of depression and/or suicidal thoughts or behavior. Healthcare providers should carefully evaluate the risks and benefits of continuing treatment with OHTUVAYRE if such events occur.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Paradoxical Bronchospasm [see Warnings and Precautions (5.2)]
- Psychiatric Events Including Suicidality [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of OHTUVAYRE was based on the pooled safety population from two randomized, double-blind, placebo-controlled trials (ENHANCE-1 and ENHANCE-2) for 24 weeks, and a 48-week cohort that assessed safety in ENHANCE-1. In these trials, a total of 975 patients received 3 mg of OHTUVAYRE twice daily administered by oral inhalation using a standard jet nebulizer [see Clinical Studies (14)]. The safety population included all patients who were randomized and received at least one dose of OHTUVAYRE or placebo.
Adverse reactions that occurred at an incidence greater than or equal to 1% in OHTUVAYRE and were more common than placebo in the pooled population are provided in Table 1.
The proportion of patients who discontinued treatment due to adverse reactions was 7.6% for the OHTUVAYRE-treated patients and 8.2% for placebo-treated patients.
Table 1. Adverse Reactions with OHTUVAYRE with incidence ≥ 1% and More Common than Placebo in Patients with COPD in the Pooled 24-Week Safety Population (ENHANCE-1 and ENHANCE-2) Adverse Reaction OHTUVAYRE
N=975
n (%)Placebo
N=574
n (%)Back pain 18 (1.8%) 6 (1.0%) Hypertension 17 (1.7%) 5 (0.9%) Urinary tract infection 13 (1.3%) 6 (1.0%) Diarrhea 10 (1.0%) 4 (0.7%) Adverse Reactions in the 48-Week Cohort
In the 48-week cohort of ENHANCE-1, 369 patients were enrolled to be treated with 3 mg OHTUVAYRE (N=280) or placebo (N=89) twice daily for 48 weeks [see Clinical Studies (14)]. The adverse reactions reported in the 48-week cohort were consistent with those observed in the pooled 24-week safety population.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on OHTUVAYRE use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage or other adverse maternal or fetal outcomes. In animal reproduction studies, administration of inhaled ensifentrine at exposures 30 times the exposure at the maximum recommended human daily inhalation dose (MRHDID) to male rats for 10 weeks prior to mating with untreated females produced increased pre- and post- implantation loss, and decreased live embryos in untreated female rats. No adverse developmental effects were observed with inhalation administration of ensifentrine to pregnant rats and rabbits during organogenesis at maternal exposures up to 79 and 9 times the exposure at MRHDID, respectively. No adverse developmental effects were observed after inhaled administration of ensifentrine to pregnant rats from the period of organogenesis through lactation at exposures up to approximately 79 times the MRHDID. (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
In a male fertility study, ensifentrine was administered to male rats at inhalation doses of 2, 6, and 16 mg/kg/day (4, 13, and 30 times the exposure at the MRHDID) for 10 weeks prior to mating to untreated females. Adverse effects at 16 mg/kg/day (30 times the exposure at the MRHDID) on reproductive performance included increased pre- and post- implantation loss, and decreased live embryos per litter in untreated females. No developmental toxicity was observed in rats at 6 mg/kg/day (13 times the exposure at the MRHDID).
In an embryo-fetal development study, pregnant rats were administered ensifentrine at inhalation doses of up to 15 mg/kg/day (79 times the exposure at the MRHDID) during the period of organogenesis from gestation Days 6 to 17. Ensifentrine did not cause adverse effects to the fetus at exposures up to 79 times the MRHDID (on an AUC basis at a maternal inhalation dose of 15 mg/kg/day).
In an embryo-fetal development study, pregnant rabbits were administered ensifentrine at inhalation doses of up to 12 mg/kg/day (9 times the exposure at the MRHDID) during the period of organogenesis from gestation Days 6 to 19. Ensifentrine did not cause adverse effects to the fetus at maternal exposures up to 9 times the MRHDID.
In a pre- and post-natal development (PPND) study, pregnant rats were administered ensifentrine at inhalation doses of up to 19 mg/kg/day (approximately 79 times the exposure at the MRHDID) from gestation day 6 to lactation/post-partum day 24. No adverse effects were observed in the offspring exposed daily from birth (in utero) through lactation at maternal pulmonary deposited doses up to approximately 79 times the MRHDID.
8.2 Lactation
Risk Summary
There are no data on the presence of ensifentrine in human milk, the effects on the breastfed child, or the effects on milk production. There are no data from animal studies on the presence of ensifentrine in milk.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for OHTUVAYRE and any potential adverse effects on the breastfed child from OHTUVAYRE or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of OHTUVAYRE have not been established in pediatric patients.
8.5 Geriatric Use
There were 852 patients aged 65 years and older in ENHANCE-1 and ENHANCE-2 for COPD [see CLINICAL STUDIES (14)]. Of the total number of patients randomized to receive OHTUVAYRE in these trials, 534 (55%) were 65 years of age and older, while 84 (9%) were 75 years and older. No overall differences in safety or effectiveness of OHTUVAYRE have been observed between these patients and younger adult patients, but greater sensitivity of some older individuals cannot be ruled out.
8.6 Hepatic Impairment
Ensifentrine systemic exposure increased by 2.3-fold in subjects with moderate or severe hepatic impairment compared with healthy subjects [see Clinical Pharmacology (12.3)]. Use OHTUVAYRE with caution in patients with hepatic impairment.
8.7 Renal Impairment
No dosage adjustment in patients with mild or moderate renal impairment is required. Patients with severe renal impairment have not been evaluated [see Clinical Pharmacology (12.3)].
- 10 OVERDOSAGE
-
11 DESCRIPTION
OHTUVAYRE (ensifentrine) is a sterile, yellow to pale yellow aqueous inhalation suspension of ensifentrine for oral inhalation. Ensifentrine, the active component of OHTUVAYRE, is an inhibitor of phosphodiesterases 3 and 4 (PDE3 and PDE4). The chemical name for ensifentrine is N-(2-{(2E)-9,10-dimethoxy-4-oxo-2-[(2,4,6-trimethylphenyl)imino]-6,7-dihydro-2H-pyrimido[6,1-a]isoquinolin-3(4H)-yl}ethyl)urea; its structural formula is:
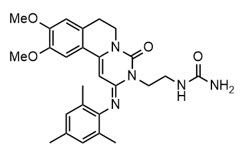
Ensifentrine has a molecular weight of 477.56 and its empirical formula is C26H31N5O4. Ensifentrine is a yellow to pale yellow crystalline powder which is practically insoluble in water.
OHTUVAYRE is supplied as 2.5 mL of sterile ensifentrine (1.2 mg/mL) suspension packaged in a unit-dose low-density polyethylene ampule overwrapped in a sealed foil pouch. Each unit-dose ampule contains 3 mg ensifentrine suspended in a pH 6.7 aqueous solution containing dibasic sodium phosphate, monobasic sodium phosphate, polysorbate 20, sodium chloride, sorbitan monolaurate and water for injection.
The ampule containing OHTUVAYRE should be shaken vigorously to ensure complete resuspension of the active ingredient immediately prior to administration by nebulization. Like all other nebulized treatments, the amount delivered to the lungs will depend on patient factors, the nebulization system used, and compressor performance.
Using the PARI LC Sprint® jet nebulizer attached to a PARI Vios Pro® compressor under in vitro conditions, the mean delivered dose from the mouthpiece was approximately 933 micrograms (31% of label claim) at a mean flow rate of approximately 5 liters per minute. The mean nebulization time was approximately 7 minutes. The mass median aerodynamic diameter (MMAD) of the nebulized particles/droplets using the PARI LC Sprint® jet nebulizer attached to a PARI Vios Pro® compressor (flow rate approximately 15 L per minute, nebulization time approximately 10 minutes) is 5.82 microns (geometric standard deviation = 1.97) with a typical fine particle dose (mass of aerosolized drug < 5 microns) of approximately 614 micrograms, as determined using the Next Generation Impactor (NGI) method, based on USP <601>. OHTUVAYRE should only be administered via a standard jet nebulizer connected to an air compressor with an adequate airflow and equipped with a mouthpiece.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Ensifentrine is a small molecule that is an inhibitor of the PDE3 and PDE4 enzymes. PDE3 primarily hydrolyzes the second-messenger molecule cyclic adenosine monophosphate (cAMP) but is also capable of hydrolyzing cyclic guanosine monophosphate (cGMP). PDE4 hydrolyzes cAMP only. Inhibition of PDE3 and PDE4 results in accumulation of intracellular levels of cAMP and/or cGMP, resulting in various downstream signalling effects.
12.3 Pharmacokinetics
Exposure to ensifentrine increased approximately 1.4-fold greater than dose proportional following a dose 3 times the recommended dosage. Steady-state was attained by Day 3 following twice-daily dosing. Population pharmacokinetic analysis predicts accumulation of ensifentrine of 1.3 and 1.4-fold for Cmax and AUC in healthy subjects and 1.4 and 1.5-fold for Cmax and AUC in subjects with COPD. Population pharmacokinetic analysis indicates that relative bioavailability in subjects with COPD is approximately 35% lower when compared to healthy subjects. Exposure to ensifentrine was associated with high inter-subject variability.
Absorption
Following inhaled administration of OHTUVAYRE in healthy subjects and subjects with COPD, ensifentrine Cmax was attained around 0.6 to 1.5 hours after dosing.
A randomized, 2-period, cross-over study assessing systemic exposure following inhalation of 2 times the recommended dose of ensifentrine with and without charcoal block demonstrated that the majority of an inhaled dose (approximately 90%) is delivered to the lung from which it is absorbed.
Distribution
Apparent central and peripheral volume of distribution for ensifentrine in healthy subjects were 2700 L and 1820 L, respectively, as estimated in population PK analysis. In patients with COPD, apparent central and peripheral volumes were estimated as 8150 L and 5490 L, respectively.
In vitro plasma protein binding of ensifentrine is approximately 90%.
Elimination
Following twice-daily administration for 6 days, terminal elimination half-life ranged from 10.6 to 12.6 hours in healthy subjects and subjects with COPD (1.5 mg to 12 mg twice daily).
Metabolism
Following administration of a single nebulized dose, 8 times the recommended dose of ensifentrine, unchanged ensifentrine was identified as the major drug-related component in human plasma, accounting for 96 and 99% of the drug-related material identified in Tmax and time-normalized (0-24 h) plasma samples, respectively.
The primary metabolic routes for ensifentrine are oxidative (hydroxylation, O-demethylation) followed by conjugation (e.g., glucuronidation).
In vitro results indicate that, at physiologically relevant concentrations, ensifentrine was predominantly metabolized by CYP2C9 and to a lesser extent by CYP2D6.
Specific Populations
Population pharmacokinetic analysis showed no evidence of a clinically significant effect of demographic covariates such as age (18 to 80 years), sex (56% male), ethnicity (Hispanic, Non-Hispanic), race (white, black) and weight (42 to 180 kg) on ensifentrine pharmacokinetics.
Patients with Renal Impairment
A dedicated study with OHTUVAYRE evaluating the effect of renal impairment on the pharmacokinetics of ensifentrine was not conducted.
The effect of renal impairment on the exposure to ensifentrine for up to 24 weeks was evaluated in a population pharmacokinetic analysis. Estimated glomerular filtration rate (eGFR) varied from 25.5 to 191 mL/min representing a range of moderate to no renal impairment. While continuous covariates of renal function did not show a significant correlation with ensifentrine exposure, categorical characterization of renal function indicated a 25% mean reduction in the apparent clearance in subjects with moderate renal impairment. The pharmacokinetics of ensifentrine in severe renal impairment (creatine clearance <30 mL/min) or subjects with end-stage renal disease have not been evaluated.
Patients with Hepatic Impairment
The pharmacokinetics of ensifentrine were evaluated in subjects with moderate (Child-Pugh Class B) (N=10) to severe (Child-Pugh Class C) (N=2) hepatic impairment. Ensifentrine Cmax and AUCinf were approximately 2.3-fold and 2.2-fold higher in subjects with moderate hepatic impairment compared with healthy controls. Ensifentrine Cmax and AUCinf were approximately 1.2-fold and 2.3-fold higher in subjects with severe hepatic impairment compared with healthy controls (N=7).
Population PK analysis did not identify markers of liver function (ALT, AST, bilirubin, and ALP) as a significant covariate for exposure of ensifentrine.
Drug Interaction Studies
Effect of Other Drugs on OHTUVAYRE
Effect of OHTUVAYRE on Other Drugs
In Vitro Studies
Ensifentrine and Cytochrome P450: At therapeutically relevant concentrations, ensifentrine does not inhibit CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, or CYP3A4.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
A two-year inhalation study in Han Wistar rats and a 6-month oral study in Tg.rasH2 transgenic mice were conducted to assess the carcinogenic potential of ensifentrine. No evidence of tumorigenicity was observed in male and female rats at an exposure approximately 40 times the MRHDID. No evidence of tumorigenicity was observed in male and female Tg.rasH2 mice at oral doses up to 80 mg/kg/day, the highest dose tested.
Ensifentrine was negative for genotoxicity in the following assays: in vitro Ames test for bacterial gene mutation, in vivo comet test with rats, or in vivo micronucleus assay with mice.
In a male fertility study, ensifentrine was administrated to male rats at inhalation doses of 2, 6, and 16 mg/kg/day (4, 13, and 30 times the exposure at the MRHDID) for 10 weeks prior to mating to untreated females. Male rats had decreased sperm motility and increased abnormal sperm morphology at an inhalation dose of 16 mg/kg/day (approximately 30 times the exposure at the MRHDID). Decreased sperm counts in the testis were observed at all doses. Atrophy/degeneration in the testis and intraluminal germ cell debris in the epididymis were observed at doses of 6 (13 times the exposure at the MRHDID) and 16 mg/kg/day (30 times the exposure at the MRHDID). Additional adverse effects at 16 mg/kg/day on reproductive performance included decreased mating index and decreased fertility index. The sperm counts, sperm motility, and sperm morphology were reversible at the end of a 4-week treatment-free period. Atrophy/degeneration in the testis and intraluminal germ cell debris in the epididymis were not present at the end of a 4-week treatment-free period. In a female fertility study, ensifentrine was administrated to female rats at inhalation doses of up to 18 mg/kg/day from two weeks prior to mating to 7 days after mating. Ensifentrine had no effect on female fertility and reproductive performance indices up to 18 mg/kg/day (31 times the exposure at the MRHDID).
-
14 CLINICAL STUDIES
The efficacy of OHTUVAYRE was evaluated in two 24-week randomized, double-blind, placebo-controlled, parallel-group clinical trials (ENHANCE-1 [NCT04535986] and ENHANCE-2 [NCT04542057]). The two trials enrolled a total of 1553 adults with moderate to severe COPD.
ENHANCE-1 enrolled a total of 763 patients randomized 5:3 to receive 3 mg of OHTUVAYRE administered by oral inhalation via standard jet nebulizer such as PARI LC Sprint® [see Description (11)], or placebo. Patients in ENHANCE-1 had a mean age of 65 years (range: 41 to 80 years), were 58% male, 90% White, 3% Black/African American, 3% Asian, 0.1% Other race, and 3% Hispanic or Latino ethnicity. Patients had a mean smoking history of 41 pack-years and 57% were current smokers, and 25% of patients reported exacerbations of COPD within the 15 months prior to the study. At screening, the mean post-bronchodilator percent predicted FEV1 was 52% (range: 27% to 85%), and the mean post-bronchodilator FEV1/FVC ratio was 0.52 (range: 0.22 to 0.71). In addition, 68% of patients were taking concurrent therapy: 30% taking concurrent LAMA, 18% taking concurrent LABA, and 20% taking concurrent LABA/ICS therapy throughout the trial.
ENHANCE-2 enrolled a total of 790 patients randomized 5:3 to receive 3 mg of OHTUVAYRE twice daily administered by oral inhalation via a standard jet nebulizer such as PARI LC Sprint® [see Description (11)] or placebo. Patients in ENHANCE-2 had a mean age of 65 years (range: 40 to 80 years), were 52% female, 95% White, 4% Black/African American, 0.3% Asian, 0.5% Other race, and 5% Hispanic or Latino ethnicity. Patients had a mean smoking history of 42 pack-years and 55% were current smokers, and 21% of patients reported exacerbations of COPD within the 15 months prior to the study. At screening, the mean post-bronchodilator percent predicted FEV1 was 51% (range: 23% to 81%), and the mean post-bronchodilator FEV1/FVC ratio was 0.52 (range: 0.24 to 0.71). In addition, 55% of patients were taking concurrent therapy: 33% taking concurrent LAMA, 7% taking concurrent LABA, and 15% taking concurrent LABA/ICS therapy throughout the trial.
The primary endpoint for ENHANCE-1 and ENHANCE-2 was the change from baseline in FEV1 AUC0-12h post dose at Week 12. In both trials, OHTUVAYRE demonstrated a statistically significant improvement in FEV1 AUC0-12h compared to placebo. Refer to Table 2 for the primary endpoint results.
Table 2. Least Squares (LS) Mean Change from Baseline in FEV1 AUC0-12h (mL) at Week 12 in ENHANCE-1 and ENHANCE-2 ENHANCE-1 ENHANCE-2 OHTUVAYRE
(N=479)Placebo
(N=284)OHTUVAYRE
(N=499)Placebo
(N=291)CI, confidence interval; LS, least squares; N= number of all enrolled patients; n = number of patients who received at least one dose of study drug and had non-missing baseline FEV1 value. n 477 282 498 291 LS Mean (95% CI) 61
(25, 97)-26
(-64, 13)48
(30, 66)-46
(-70, -22)LS Mean Difference from Placebo (95% CI) 87
(55, 118)- 94
(65, 124)- p-value <0.0001 - <0.0001 - In ENHANCE-1 and ENHANCE-2, serial spirometry was performed over 12 hours in all patients at baseline and Week 12. Serial spirometry data for ENHANCE-1 at Week 12 are shown in Figure 1.
Figure 1. Mean FEV1 (mL) Change from Baseline over 12 hours at Week 12 (ENHANCE-1)
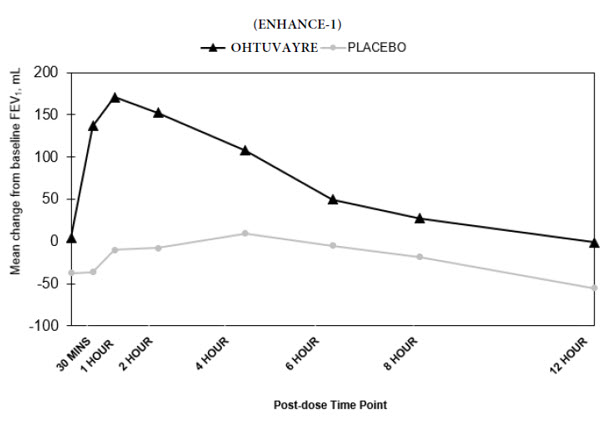
Trough FEV1 was defined as the last FEV1 value collected prior to the morning dose. The mean morning trough FEV1 improvement at Week 12 relative to placebo was 35 mL (95% CI: 14, 68) and 49 mL (95% CI: 19, 80) in ENHANCE-1 and ENHANCE-2, respectively, which was statistically significant in ENHANCE-1, and not statistically significant in ENHANCE-2 due to failure higher in the testing hierarchy.
Health-Related Quality of Life
The St. George's Respiratory Questionnaire (SGRQ) was assessed in ENHANCE-1 and ENHANCE-2. In ENHANCE-1, the SGRQ responder rate (defined as an improvement in score of 4 or more as threshold) for OHTUVAYRE at Week 24 was 58.2% compared to 45.9% for placebo [Odds Ratio: 1.49; 95% CI: 1.07, 2.07]. In ENHANCE-2, the SGRQ responder rate for OHTUVAYRE at Week 24 was 45.4% compared to 50.3% for placebo [Odds Ratio: 0.92; 95% CI: 0.66, 1.29].
-
16 HOW SUPPLIED/STORAGE AND HANDLING
OHTUVAYRE (ensifentrine) 3 mg/2.5 mL inhalation suspension is a sterile aqueous suspension in a unit-dose low-density polyethylene ampule. OHTUVAYRE is supplied as:
- Carton of 60: 60 pouches of 1 unit-dose ampule (NDC: 83034-003-60)
- Carton of 60: 12 pouches of 5 unit-dose ampules (NDC: 83034-003-65)
Ampules are overwrapped in a sealed foil pouch. The ampule containing OHTUVAYRE should be shaken vigorously to ensure complete resuspension of the active ingredient immediately prior to use. The used ampule and any residual content should be discarded after use.
Store OHTUVAYRE in the protective foil pouch. Only remove an ampule from foil pouch immediately before use. For pouches of 5 ampules, remove one ampule and place the remaining ampules back into the foil pouch until next use. Once the foil pouch is opened, discard ampules if not used within 14 days. Store at controlled room temperature (68°F to 77°F [20°C to 25°C], excursions permitted from 59ºF to 86ºF [15ºC to 30ºC]) [See USP Controlled Room Temperature], maintaining the orientation indicated on the carton. Protect from direct sunlight and excessive heat. Do not freeze.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Not for Treatment of Acute Symptoms of Bronchospasm
Inform patients that OHTUVAYRE is not meant to relieve acute symptoms of COPD and extra doses should not be used for that purpose. Advise patients to treat acute symptoms with an inhaled, short-acting beta2-agonist such as albuterol [see Warnings and Precautions (5.1)]. Provide patients with such medicine and instruct them in how it should be used.
Instruct patients to seek medical attention immediately if they experience any of the following:
- Decreasing effectiveness of inhaled, short-acting beta2-agonists
- Need for more inhalations than usual of inhaled, short-acting beta2-agonists
- Significant decrease in lung function as outlined by the health care provider
Paradoxical Bronchospasm
As with other inhaled medicines, OHTUVAYRE can cause paradoxical bronchospasm. If paradoxical bronchospasm occurs, instruct patients to discontinue OHTUVAYRE [see Warnings and Precautions (5.2)].
Psychiatric Events Including Suicidality
Advise patients, caregivers, and families to be alert for the emergence or worsening of insomnia, anxiety, depression, suicidal thoughts or other mood changes, and if such changes occur to contact their healthcare provider so that the risks and benefits of continuing treatment with OHTUVAYRE may be considered [see Warnings and Precautions (5.3)].
Administration Instructions
Instruct patients that OHTUVAYRE should only be administered via a standard jet nebulizer [see Instructions for Use]. Patients should be instructed not to inject or swallow OHTUVAYRE. Patients should be instructed not to mix other medications with OHTUVAYRE in a nebulizer.
Inform patients to use the contents of one ampule of OHTUVAYRE orally inhaled twice daily, once in the morning and once in the evening. Instruct patients to vigorously shake OHTUVAYRE to ensure complete resuspension of the active ingredient immediately prior to use [see Dosage and Administration (2)].
- SPL UNCLASSIFIED SECTION
-
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration. Approved: 07/2025
PIL002PATIENT INFORMATION
OHTUVAYRE (OH-too-vare)
(ensifentrine)
inhalation suspension, for oral inhalationImportant: For oral inhalation only. Do not swallow or inject OHTUVAYRE. What is OHTUVAYRE? - OHTUVAYRE is a prescription medicine used to treat chronic obstructive pulmonary disease (COPD) in adults. COPD is a chronic (long-term) lung disease that includes chronic bronchitis, emphysema, or both.
- OHTUVAYRE is an inhibitor of phosphodiesterases 3 and 4 (PDE3 and PDE4).
- Decreasing PDE3 activity helps the muscles around the airways in your lungs stay relaxed to prevent symptoms such as wheezing, coughing, chest tightness, and shortness of breath, which can happen when muscles around the airway tighten, making it hard to breathe. Decreasing PDE4 activity helps to decrease inflammation in the lungs. Inflammation in the lungs can lead to breathing problems.
- OHTUVAYRE is used to improve symptoms of COPD for better breathing and to reduce the number of flare-ups (the worsening of your COPD symptoms for several days).
- OHTUVAYRE is used long-term as 1 ampule of OHTUVAYRE, 2 times each day (1 in the morning and 1 in the evening) inhaled through your nebulizer that is fitted with a mouthpiece.
- OHTUVAYRE is not used to relieve sudden breathing problems and will not replace an inhaled rescue medicine.
- OHTUVAYRE should not be used in children. It is not known if OHTUVAYRE is safe and effective in children.
Do not use OHTUVAYRE if you have had an allergic reaction to ensifentrine or any of the ingredients in OHTUVAYRE. See "What are the ingredients in OHTUVAYRE?" at the end of this Patient Information leaflet for a complete list of ingredients in OHTUVAYRE. Before taking OHTUVAYRE, tell your healthcare provider about all your medical conditions, including if you: - have or have had a history of mental health problems including depression and suicidal behavior.
- have liver problems.
- are pregnant or plan to become pregnant. It is not known if OHTUVAYRE may harm your unborn baby.
- are breastfeeding. It is not known if the medicine in OHTUVAYRE passes into your breast milk and if it can harm your baby.
How should I use OHTUVAYRE?
Read the step-by-step instructions for using OHTUVAYRE at the end of this Patient Information leaflet.- OHTUVAYRE is only for use with a nebulizer.
- Do not use OHTUVAYRE unless your healthcare provider has taught you how to use it with your nebulizer and you understand how to use it correctly.
- Use OHTUVAYRE exactly as your healthcare provider tells you to use it. Do not use OHTUVAYRE more often than prescribed.
- OHTUVAYRE is taken as a breathing treatment by oral (mouth) inhalation and should be used with a standard jet nebulizer with a mouthpiece connected to an air compressor.
- Do not mix other medicines with OHTUVAYRE in your nebulizer.
- Shake the OHTUVAYRE ampule vigorously immediately before using it. When shaken, OHTUVAYRE will appear cloudy and yellow to pale yellow in color.
- Use 1 ampule of OHTUVAYRE 2 times a day, 1 time in the morning and 1 time in the evening. Do not use more than 1 ampule of OHTUVAYRE in a single treatment. Do not use more than 2 ampules of OHTUVAYRE a day.
- If you use too much OHTUVAYRE, call your healthcare provider or go to the nearest hospital emergency room right away if you have any unusual symptoms, such as increased heart rate or headaches.
- OHTUVAYRE does not relieve sudden symptoms of COPD and you should not use extra doses of OHTUVAYRE to relieve these sudden symptoms. Always have an inhaled rescue medicine with you to treat sudden symptoms. If you do not have an inhaled rescue medicine, call your healthcare provider to have one prescribed for you.
- Call your healthcare provider or get emergency medical care right away if:
- your breathing problems get worse.
- you need to use your inhaled rescue medicine more often than usual.
- your inhaled rescue medicine does not relieve your symptoms.
- Do not stop using OHTUVAYRE, even if you are feeling better, unless your healthcare provider tells you to, because your symptoms might get worse.
- Throw away the OHTUVAYRE ampules immediately after use. Due to their small size, the ampules pose a danger of choking to young children.
What are the possible side effects of OHTUVAYRE?
OHTUVAYRE can cause serious side effects, including:- Sudden breathing problems immediately after inhaling your medicine. If you have sudden breathing problems immediately after inhaling your medicine, stop using OHTUVAYRE and call your healthcare provider right away or go to the nearest hospital emergency room right away.
- Mental health problems including suicidal thoughts and behavior. You may experience mood or behavior changes when taking OHTUVAYRE. Call your healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you:
- thoughts of suicide or dying
- attempt to commit suicide
- trouble sleeping (insomnia)
- new or worse anxiety
- new or worse depression
- acting on dangerous impulses
- other unusual changes in your behavior or mood
Most common side effects of OHTUVAYRE include: - back pain
- high blood pressure
- bladder infection
- diarrhea
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store OHTUVAYRE? - Store OHTUVAYRE at room temperature between 68°F to 77°F (20°C to 25°C). Keep OHTUVAYRE away from direct sunlight and excessive heat. Do not freeze.
- Store OHTUVAYRE in the unopened protective foil pouch and only open the foil pouch immediately before using OHTUVAYRE. For pouches of 5 ampules, remove one ampule and place the remaining ampules back into the pouch until next use.
- Throw away the used ampule and any leftover medicine after use.
- Do not use OHTUVAYRE after the expiration date provided on the foil pouch and ampule.
- Keep OHTUVAYRE and all medicines out of the reach of children.
General information about the safe and effective use of OHTUVAYRE.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use OHTUVAYRE for a condition for which it was not prescribed. Do not give OHTUVAYRE to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your healthcare provider or pharmacist for information about OHTUVAYRE that is written for health professionals.What are the ingredients in OHTUVAYRE?
Active ingredient: ensifentrine
Inactive ingredients: dibasic sodium phosphate, monobasic sodium phosphate, polysorbate 20, sodium chloride, sorbitan monolaurate and water for injection.
Manufactured for:
Verona Pharma, Inc.
Raleigh, NC, 27615
©2025. Verona Pharma plc and its affiliates
Verona Pharma, OHTUVAYRE and the Verona Pharma and OHTUVAYRE logos are all trademarks of Verona Pharma plc -
INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE
OHTUVAYRE (OH-too-vare)
(ensifentrine)
inhalation suspension, for oral inhalationThis Instructions for Use contains information on how to use OHTUVAYRE. 
To use OHTUVAYRE, you will also need a jet nebulizer machine and air compressor. OHTUVAYRE
single-dose ampuleImportant Information You Need to Know Before Using OHTUVAYRE
- For oral inhalation only
- OHTUVAYRE is used only in a standard jet nebulizer machine with a mouthpiece connected to an air compressor.
- Do not mix other medicines with OHTUVAYRE in your nebulizer.
- OHTUVAYRE comes in an ampule that is sealed in a foil pouch. Do not open the sealed pouch until you are ready to use a dose of OHTUVAYRE.
- Use your OHTUVAYRE right away after opening. For pouches of 5 ampules, place the remaining ampules back into the pouch until next use.
- Throw away the OHTUVAYRE ampules immediately after use. Due to their small size, the ampules pose a danger of choking to young children.
Preparing to Use OHTUVAYRE
- Make sure you know how to use your nebulizer machine before you use it to breathe in OHTUVAYRE.
- Read the following steps before using OHTUVAYRE. If you have any questions, ask your healthcare provider or pharmacist.
Using OHTUVAYRE
Step 1. Open Pouch: Open the foil pouch by tearing along the seam of the pouch. Remove an ampule of OHTUVAYRE from the foil pouch. For pouches of 5 ampules, remove one ampule and place the remaining ampules back into the pouch until next use.
Step 2. Shake Ampule: Shake ampule vigorously until the contents are thoroughly mixed (see Figure A). The contents will appear cloudy and yellow to pale yellow in color.
Figure A 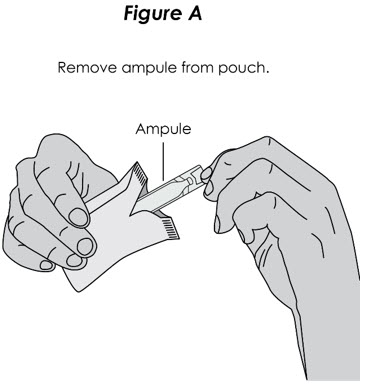
Step 3. Open Ampule: Carefully twist open the top of the ampule and use the contents right away (see Figure B).
Figure B 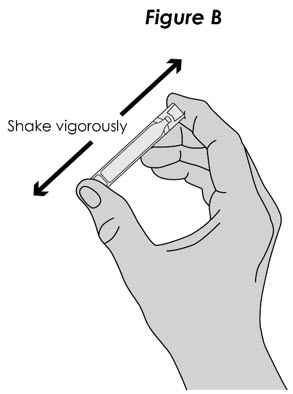
Step 4. Add Medicine: Squeeze all the medicine from the ampule into the nebulizer cup (reservoir) (see Figure C).
Figure C 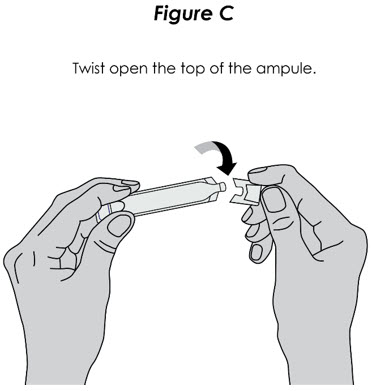
Step 5. Attach Mouthpiece: If a separate mouthpiece attachment is supplied with your nebulizer, connect the mouthpiece to the nebulizer (see Figure D).
Figure D 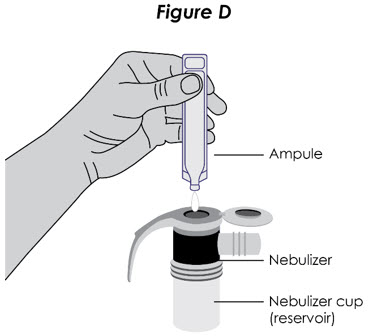
Step 6. Connect the Nebulizer to the Compressor: Firmly insert one end of the tubing to the compressor. Insert the other end of the tubing to the bottom of the nebulizer cup (reservoir) (see Figure E).
Figure E 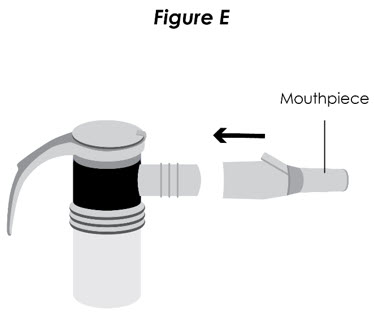
Step 7. Prepare for Treatment: Sit in a comfortable, upright position. Place the mouthpiece in your mouth and close your lips around the mouthpiece (see Figure F).
Figure F 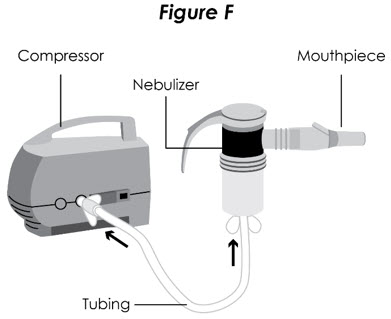
Step 8. Begin Treatment: Turn on the compressor to begin treatment.
Step 9. Breathe in Medicine: Breathe calmly, deeply, and evenly as possible until no more mist is seen in the nebulizer reservoir. Your treatment will typically take about 5 to 7 minutes. When you no longer see any mist in the nebulizer, your treatment is completed. Turn the compressor off.
Storing OHTUVAYRE and Nebulizer
- Clean and store your nebulizer. See the manufacturer's instructions that come with your nebulizer for how to clean and store your nebulizer.
- Store OHTUVAYRE ampules in the protective foil pouch in the carton at room temperature between 68°F to 77°F (20°C to 25°C) away from direct sunlight and excessive heat, in an upright position as indicated on the carton. Do not freeze.
Additional Information
Please call Verona Pharma at 1-888-672-0371 for more product information or to report problems with the product. Please call Verona Pharma at 1-888-672-0371 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch to report adverse reactions.
Manufactured for: Verona Pharma, Inc., Raleigh, NC 27615
©2025. Verona Pharma plc and its affiliates
Verona Pharma, OHTUVAYRE and the Verona Pharma and OHTUVAYRE logos are all trademarks of Verona Pharma plc
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Approved: 7/2025IFU002
-
PRINCIPAL DISPLAY PANEL - 3 mg/2.5 mL Ampule Carton
Ohtuvayre™
(ensifentrine)
Inhalation Suspension3 mg/2.5 mL
FOR ORAL INHALATION ONLY
↑ STORE UPRIGHT
NDC: 83034-003-60
Rx Only
60 UNIT-DOSE AMPULES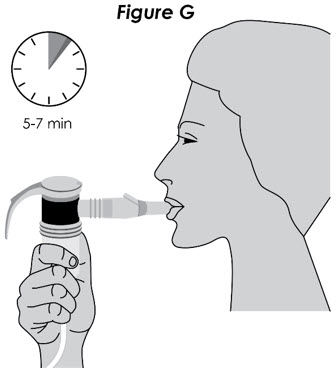
-
INGREDIENTS AND APPEARANCE
OHTUVAYRE
ensifentrine suspensionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 83034-003 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ensifentrine (UNII: 3E3D8T1GIX) (ensifentrine - UNII:3E3D8T1GIX) ensifentrine 3 mg in 2.5 mL Inactive Ingredients Ingredient Name Strength sodium chloride (UNII: 451W47IQ8X) SODIUM PHOSPHATE, DIBASIC, DIHYDRATE (UNII: 94255I6E2T) SODIUM PHOSPHATE, MONOBASIC, DIHYDRATE (UNII: 5QWK665956) polysorbate 20 (UNII: 7T1F30V5YH) sorbitan monolaurate (UNII: 6W9PS8B71J) water (UNII: 059QF0KO0R) Product Characteristics Color YELLOW (yellow to pale yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 83034-003-60 60 in 1 CARTON 06/26/2024 1 NDC: 83034-003-01 1 in 1 POUCH 1 2.5 mL in 1 AMPULE; Type 0: Not a Combination Product 2 NDC: 83034-003-65 60 in 1 CARTON 06/26/2024 2 NDC: 83034-003-05 5 in 1 POUCH 2 2.5 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA217389 06/26/2024 Labeler - Verona Pharma, Inc. (118802074) Establishment Name Address ID/FEI Business Operations Hovione FarmaCiencia SA - Sete Casas 449818434 API MANUFACTURE(83034-003) , ANALYSIS(83034-003) Establishment Name Address ID/FEI Business Operations QC Pharma 449831130 ANALYSIS(83034-003) Establishment Name Address ID/FEI Business Operations The Ritedose Corporation 837769546 MANUFACTURE(83034-003) , PACK(83034-003) , LABEL(83034-003) , ANALYSIS(83034-003) Establishment Name Address ID/FEI Business Operations The Ritedose Corporation 117131349 PACK(83034-003) , LABEL(83034-003) Establishment Name Address ID/FEI Business Operations Quality Chemical Laboratories ("QCL") 071344167 ANALYSIS(83034-003)
Trademark Results [Ohtuvayre]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 OHTUVAYRE 98494784 not registered Live/Pending |
Verona Pharma PLC 2024-04-11 |
 OHTUVAYRE 98450540 not registered Live/Pending |
Verona Pharma PLC 2024-03-14 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.