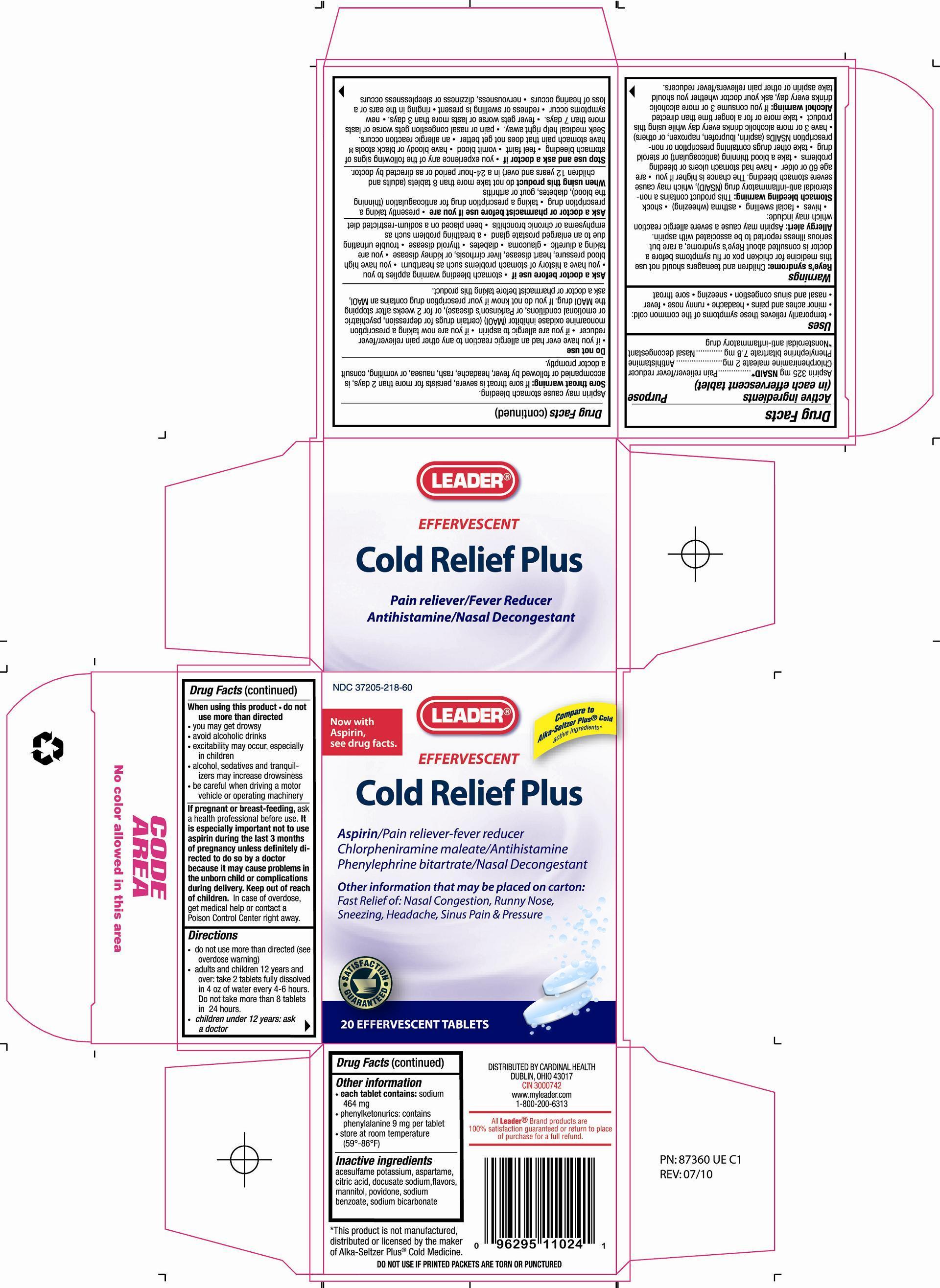
EFFERVESCENT COLD RELIEF- aspirin, chlorpheniramine maleate, phenylephrine bitartrate tablet, effervescent
Effervescent Cold Relief by
Drug Labeling and Warnings
Effervescent Cold Relief by is a Otc medication manufactured, distributed, or labeled by Cardinal Health, Inc, United Exchange Corporation, Tower Laboratories Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
ACTIVE INGREDIENT
Active Ingredients (in each effervescent tablet) Purpose
Aspirin 325 mg NSAID*.......................................... Pain reliever/fever reducer
Chlorpheniramine maleate 2 mg ............................... Antihistamine
Phenylephrine Bitartrate 7.8 mg ............................... Nasal decongestant*Nonsteroidal anti-inflammatory drug
-
WARNINGS
Warnings
Reye's syndrome: Children and teenagers should not use this drug for chicken pox or flu symptoms before a doctor is consulted about Reye's syndrome, a rare but serious illness reported to be associated with aspirin.
Allergy alert: Aspirin may cause a server allergic reaction which may include: - hives - facial swelling - asthma (wheezing) - shock
Stomach bleeding warning: This product contains a nonsteroidal anti-inflammatory drug (NSAID), which may cause severe stomach bleeding. The chance is higher if you - are age 60 or older - have had stomach ulcers or bleeding problems - take a blood thinning (anticoagulant) or steroid drug - take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others) - have 3 or more alcoholic drinks every day while using this product - take more or for a longer time than directed.
Alcohol warning: If you consume 3 or more alcoholic drinks every day, ask your doctor whether you should take aspirin or other pain relievers/fever reducers. Aspirin may cause stomach bleeding.
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
-
ASK DOCTOR
Ask a doctor before use if - stomach bleeding warning applies to you - you have a history of stomach problems, such as heartburn - you have high blood pressure, heart disease, liver cirrhosis, or kidney disease - glaucome - diabetes - thyroid disease - trouble urinating due to an enlarged prostate gland - a breathing problem such as emphysema or chronic bronchitis - you are taking a diuretic - been placed on a sodium-restricted diet.
- ASK DOCTOR/PHARMACIST
-
DO NOT USE
Do not use - if you have ever had an allergic reaction to any other pain reliever/fever reducer - if you are allergic to aspirin - if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- KEEP OUT OF REACH OF CHILDREN
- PREGNANCY OR BREAST FEEDING
- PURPOSE
- INDICATIONS & USAGE
-
STOP USE
Stop use and ask a doctor if - you experience any of the following signs of stomach bleeding - feel faint - vomit blood - have bloody stools - have stomach pain that does not get better - an allergic reaction occurs, seek medical help right away. - pain or nasal congestion gets worse or lasts more than 7 days. - fever gets worse or lasts more than 3 days. - new symptoms occur - redness or swelling is present - ringing in the ears or loss of hearing occurs - nervousness, dizziness or sleeplessness occurs.
-
WHEN USING
When using this product:
- do not take more than 8 tablets (adults and children 12 years and over) in a 24-hour period or as directed by a doctor.
- do not use more than directed
- you may get drowsy
- avoid alcoholic drinks
- excitability may occur, especially in children
- alcohol, sedatives and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery - DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
EFFERVESCENT COLD RELIEF
aspirin, chlorpheniramine maleate, phenylephrine bitartrate tablet, effervescentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 37205-218 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASPIRIN (UNII: R16CO5Y76E) (ASPIRIN - UNII:R16CO5Y76E) ASPIRIN 325 mg CHLORPHENIRAMINE MALEATE (UNII: V1Q0O9OJ9Z) (CHLORPHENIRAMINE - UNII:3U6IO1965U) CHLORPHENIRAMINE MALEATE 2 mg PHENYLEPHRINE BITARTRATE (UNII: 27O3Q5ML57) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE BITARTRATE 7.8 mg Inactive Ingredients Ingredient Name Strength ACESULFAME POTASSIUM (UNII: 23OV73Q5G9) ASPARTAME (UNII: Z0H242BBR1) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) DOCUSATE SODIUM (UNII: F05Q2T2JA0) MANNITOL (UNII: 3OWL53L36A) POVIDONE K30 (UNII: U725QWY32X) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM BICARBONATE (UNII: 8MDF5V39QO) Product Characteristics Color white Score no score Shape ROUND Size 25mm Flavor LEMON (N&A Lemon Lime Flavor FAKP193) Imprint Code CF Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 37205-218-60 20 in 1 CARTON Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 07/01/2010 Labeler - Cardinal Health, Inc (097537435) Registrant - United Exchange Corporation (840130579) Establishment Name Address ID/FEI Business Operations Tower Laboratories Ltd 869024500 manufacture(37205-218)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.