Regener-Eyes by Regenerative Processing Plant, LLC / Regenerative Process Plant
Regener-Eyes by
Drug Labeling and Warnings
Regener-Eyes by is a Otc medication manufactured, distributed, or labeled by Regenerative Processing Plant, LLC, Regenerative Process Plant. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
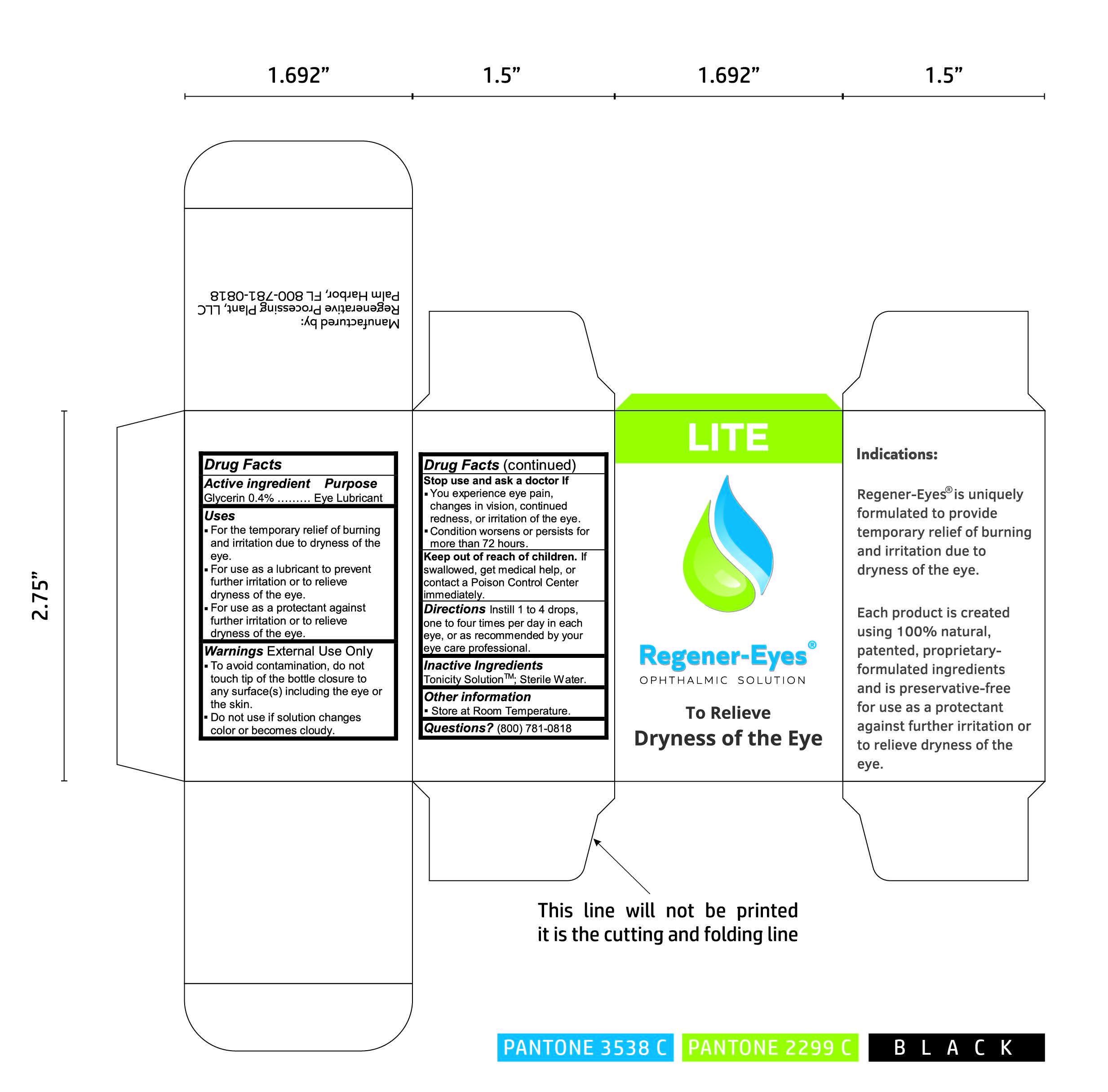
REGENER-EYES LITE- regenerative processing plant solution/ drops
Regenerative Processing Plant, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Uses
- For the temporary relief of burning and irritation due to dryness of the eye.
- For use as a lubricant to prevent further irritation or to relieve dryness of the eye.
- For use as a protectant against further irritation or to relieve dryness of the eye.
Warnings
Warnings External Use Only
- To avoid contamination, do not touch tip of the bottle closure to any surface(s) including the eye or the skin.
- Do not use if solution changes color or becomes cloudy.
Stop use and ask a doctor If
- You experience eye pain, changes in vision, continued redness, or irritation of the eye.
- Condition worsens or persists for more than 72 hours.
Keep out of reach of children
Keep out of reach of children. If swallowed, get medical help, or contact a Poison Control Center immediately.
| REGENER-EYES
LITE
regenerative processing plant solution/ drops |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Regenerative Processing Plant, LLC (079446889) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Regenerative Process Plant | 079446889 | manufacture(82305-004) , label(82305-004) , pack(82305-004) , analysis(82305-004) | |
Revised: 8/2022
Document Id: e7773258-9e14-140f-e053-2995a90a232a
Set id: e6993ab7-d042-6d71-e053-2995a90a6a14
Version: 3
Effective Time: 20220830
Trademark Results [Regener-Eyes]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 REGENER-EYES 86753016 4941280 Live/Registered |
MAM HOLDINGS OF WEST FLORIDA, LLC 2015-09-10 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.