Freshkote Preservative Free Lubricant Eye Drops
Freshkote Preservative Free Lubricant Eye Drops by
Drug Labeling and Warnings
Freshkote Preservative Free Lubricant Eye Drops by is a Otc medication manufactured, distributed, or labeled by Excelvision. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
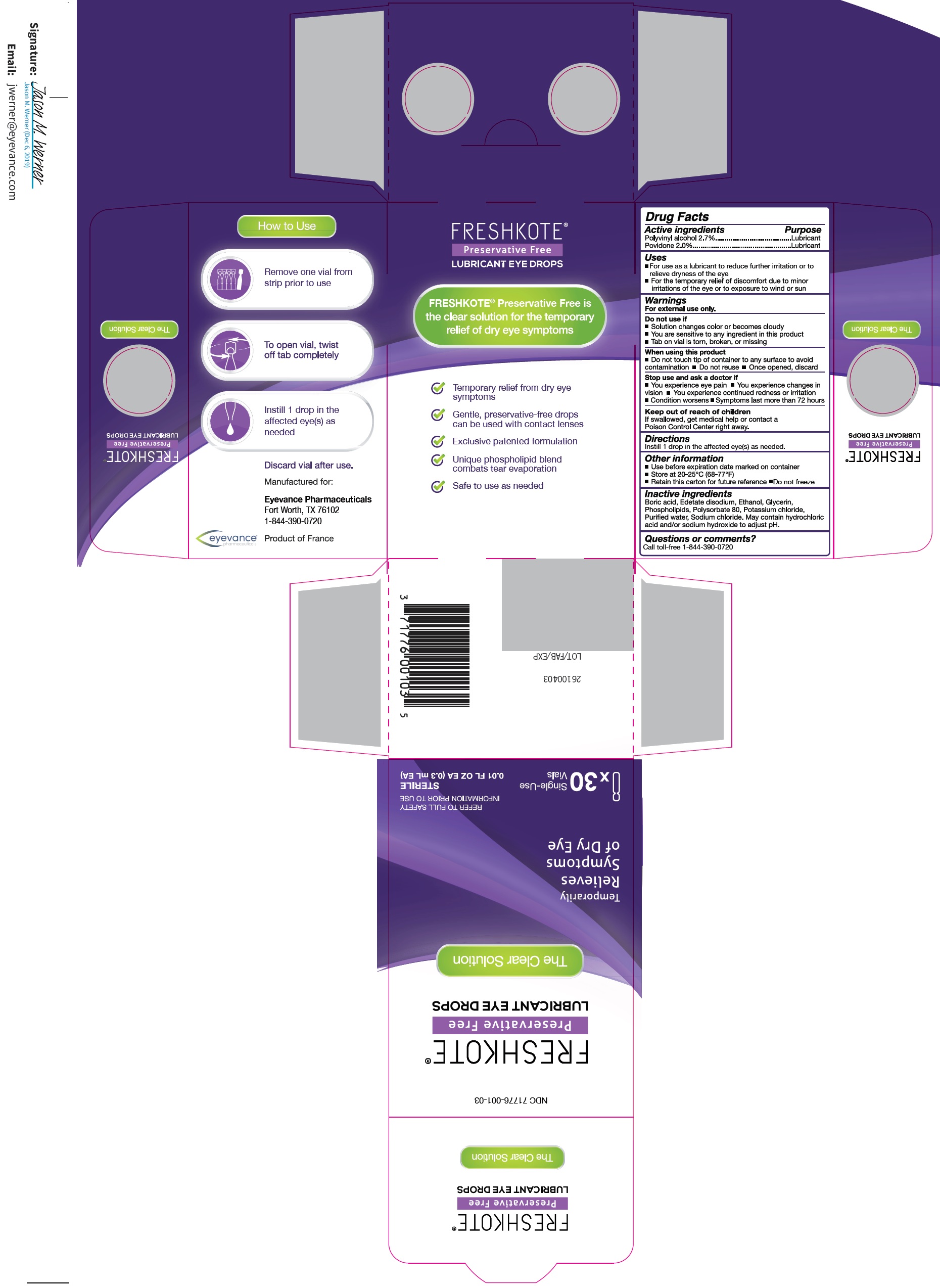
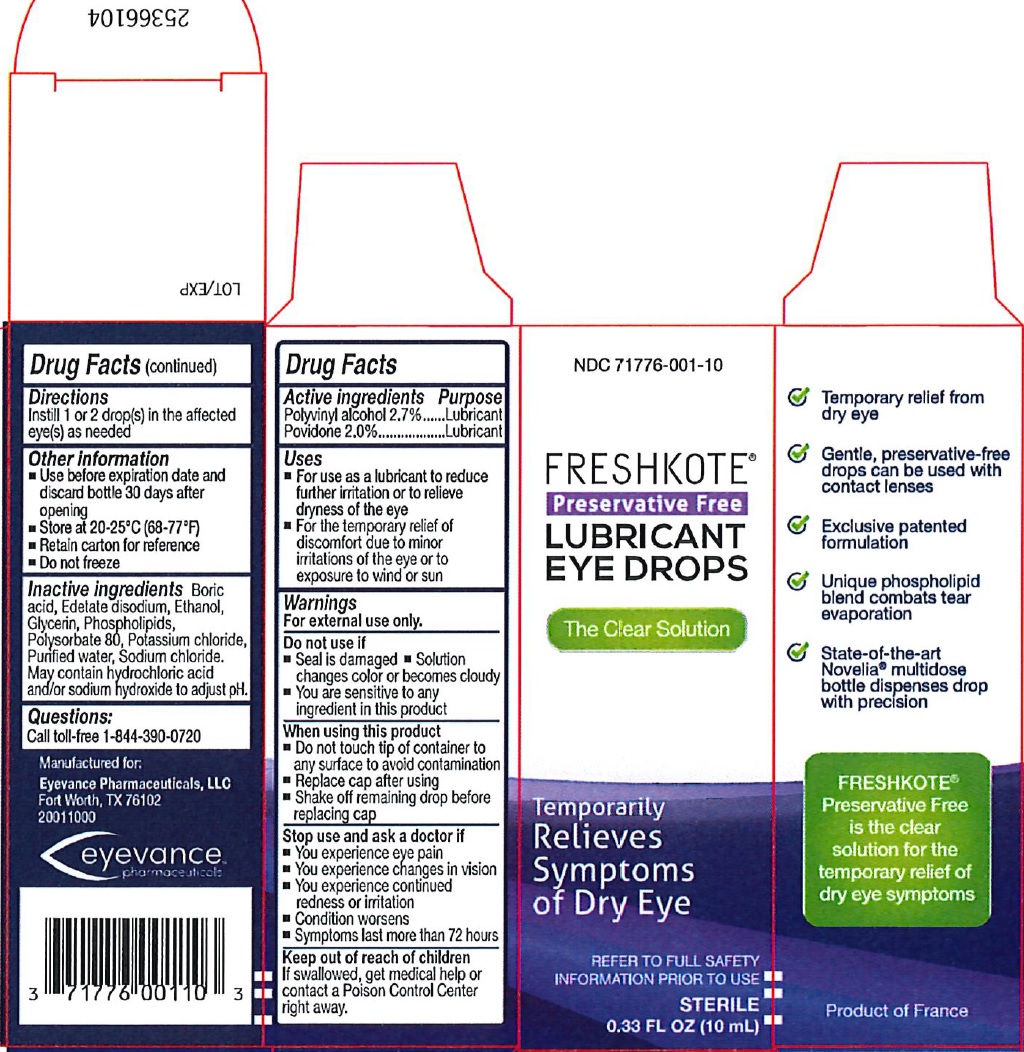
FRESHKOTE PRESERVATIVE FREE LUBRICANT EYE DROPS- polyvinyl alcohol, unspecified, povidone solution
Excelvision
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Freshkote Preservative Free Lubricant Eye Drops
Uses
- For use as a lubricant to reduce further irritation or to relieve dryness of the eye
- For the temporary relief of discomfort due to minor irritations of the eye or to exposure to wind or sun
Warnings
For external use only.
Do not use if
- Solution changes color or becomes cloudy
- You are sensitive to any ingredient in this product
- Tab on vial is torn, broken, or missing
When using this product
- Do not touch tip of container to any surface to avoid contamination
- Do not reuse
- Once opened, discard
Other information
- Use before expiration date marked on container
- Store at 20-25°C (68-77°F)
- Retain this carton for future referece
- Do not freeze
| FRESHKOTE PRESERVATIVE FREE LUBRICANT EYE DROPS
polyvinyl alcohol, unspecified, povidone solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Excelvision (274234566) |
Revised: 4/2021
Document Id: bf8f89d0-0c43-542f-e053-2995a90a3dee
Set id: e6b73558-ea08-44b1-a616-421d9f8423e3
Version: 1
Effective Time: 20210409
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.