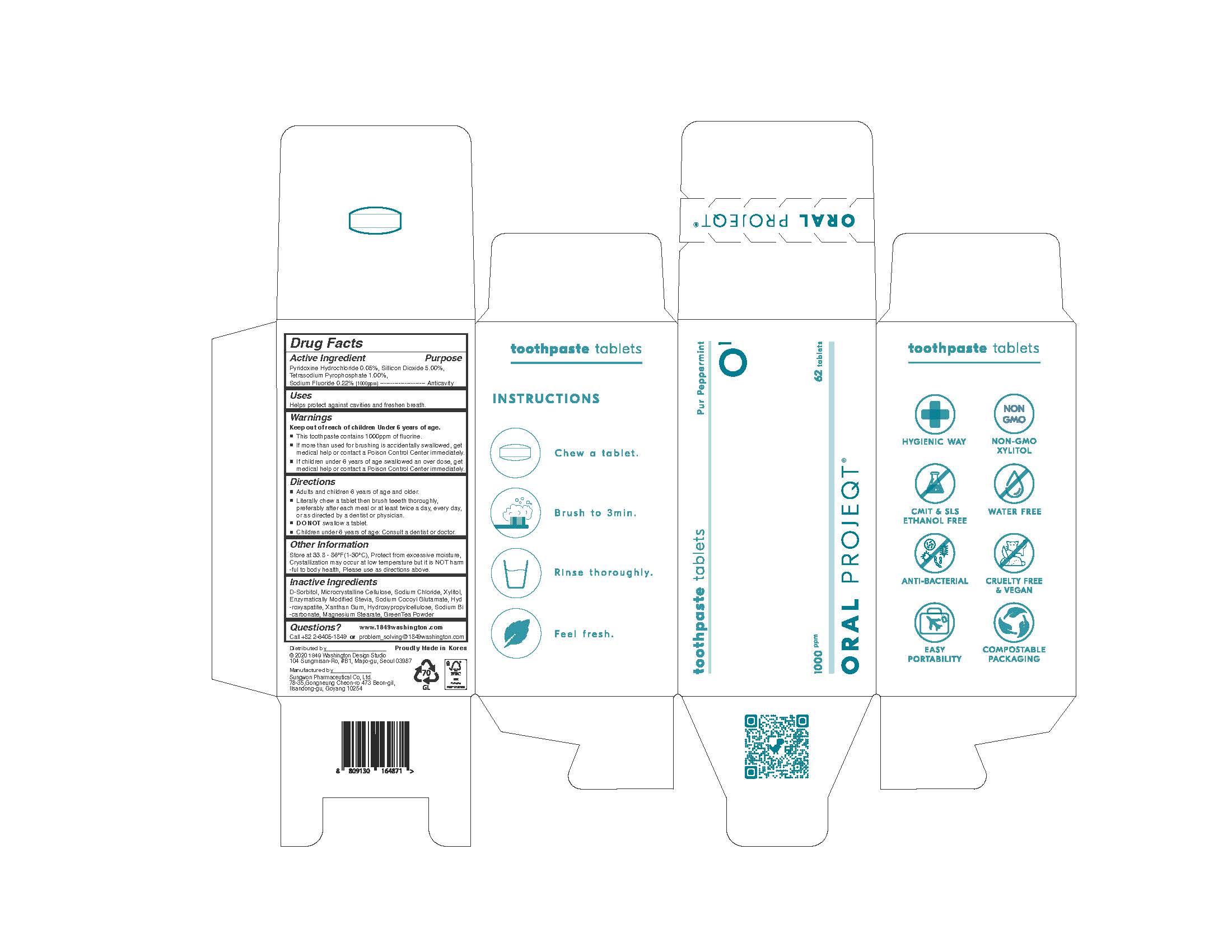
ORAL PROJEQT O1 TOOTHPASTETABLETS- sodium fluoride tablet
ORAL PROJEQT O1 TOOTHPASTETABLETS by
Drug Labeling and Warnings
ORAL PROJEQT O1 TOOTHPASTETABLETS by is a Otc medication manufactured, distributed, or labeled by Sungwon Pharmaceutical Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
D-Sorbitol, Microcrystalline Cellulose, Sodium Chloride, Xylitol, Enzymatically Modified Stevia, Sodium Cocoyl Glutamate, GreenTea Flavor Powder, Combined Flavor(Coolmint Flavor Powder), Combined Flavor(Peppermint Flavor Powder), L-Menthol, Lemon Juice, Aloe Extract Powder, Tea Extract, Hydroxyapatite, Xanthan Gum, Hydroxypropylcellulose, Sodium Bicarbonate, Magnesium Stearate
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
-
WARNING
Keep out of reach of children Under 6 years of age.
If more than used for brushing is accidentally swallowed, get
medical help or contact a Poison Control Center immediately.
This toothpaste contains 1000ppm of fluorine.
If children under 6 years of age swallowed an over dose, get
medical help or contact a Poison Control Center immediately. - USES
- INDICATION & USAGE SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ORAL PROJEQT O1 TOOTHPASTETABLETS
sodium fluoride tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 76058-500 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.22 g SILICON DIOXIDE (UNII: ETJ7Z6XBU4) (SILICON DIOXIDE - UNII:ETJ7Z6XBU4) SILICON DIOXIDE 5 g SODIUM PYROPHOSPHATE (UNII: O352864B8Z) (PYROPHOSPHORIC ACID - UNII:4E862E7GRQ) SODIUM PYROPHOSPHATE 1 g PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE HYDROCHLORIDE 0.05 g Inactive Ingredients Ingredient Name Strength XYLITOL (UNII: VCQ006KQ1E) SORBITOL (UNII: 506T60A25R) Product Characteristics Color white Score no score Shape ROUND Size 11mm Flavor Imprint Code None Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 76058-500-01 62 in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 08/01/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 08/01/2022 Labeler - Sungwon Pharmaceutical Co., Ltd. (689787898) Registrant - Sungwon Pharmaceutical Co., Ltd. (689787898) Establishment Name Address ID/FEI Business Operations Sungwon Pharmaceutical Co., Ltd. 689787898 manufacture(76058-500)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.